⇨ FDA’s briefing docs released today! 🔔
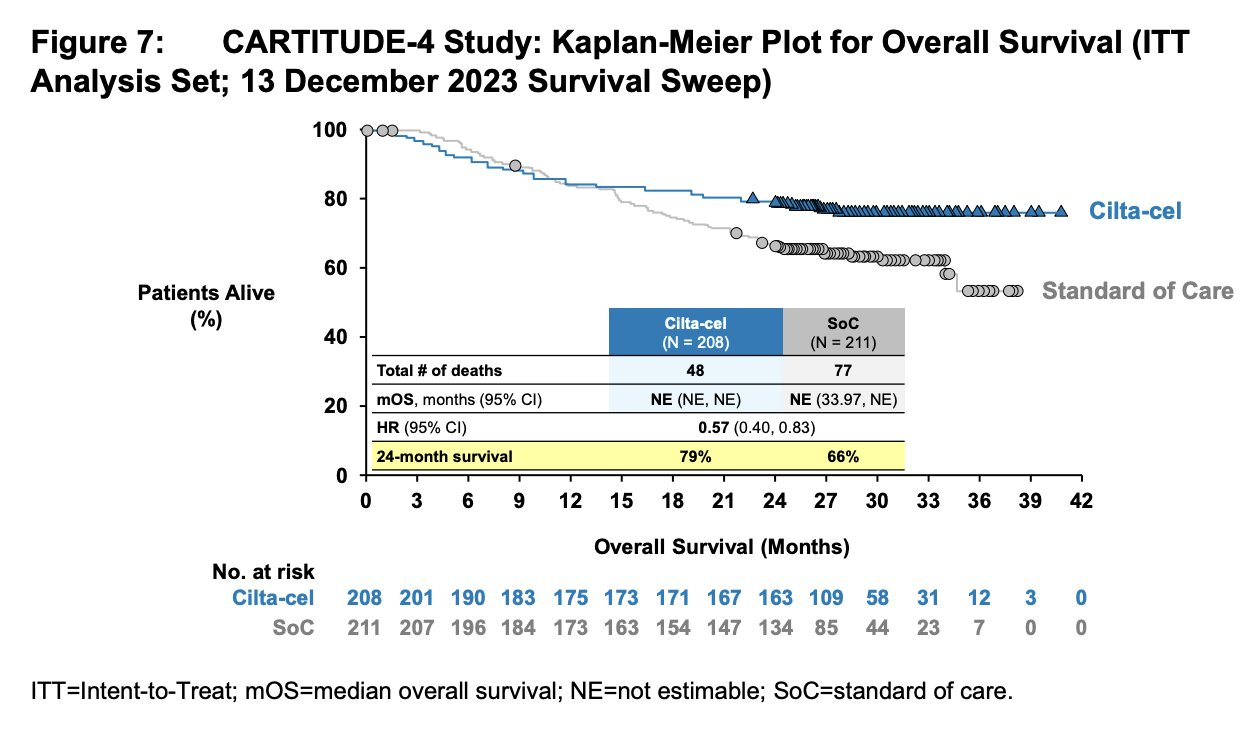
⚠️ Early deaths in cilta-cel arm raise FDA concern despite PFS benefit... 🤔
$LEGN trending up ~6% after doc release 📈
ℹ️ CARVYKTI (ciltacabtagene autoleucel)
⇒ multiple myeloma
⇒ CARTITUDE-4 study
⇒ sBLA
⇒ PDUFA: 4/5/24
❓AdCom Discussion & Question:
Discussion Topic:
⇒ Discuss whether the results of CARTITUDE-4 provided in the supplemental application are sufficient to support a positive risk-benefit assessment of ciltacabtagene-autoleucel for the proposed indication. Specifically, is the risk of early death associated with cilta-cel treatment acceptable in the context of the clinical benefit.
Voting Question:
⇒ Is the risk-benefit assessment for ciltacabtagene-autoleucel for the proposed indication, favorable?