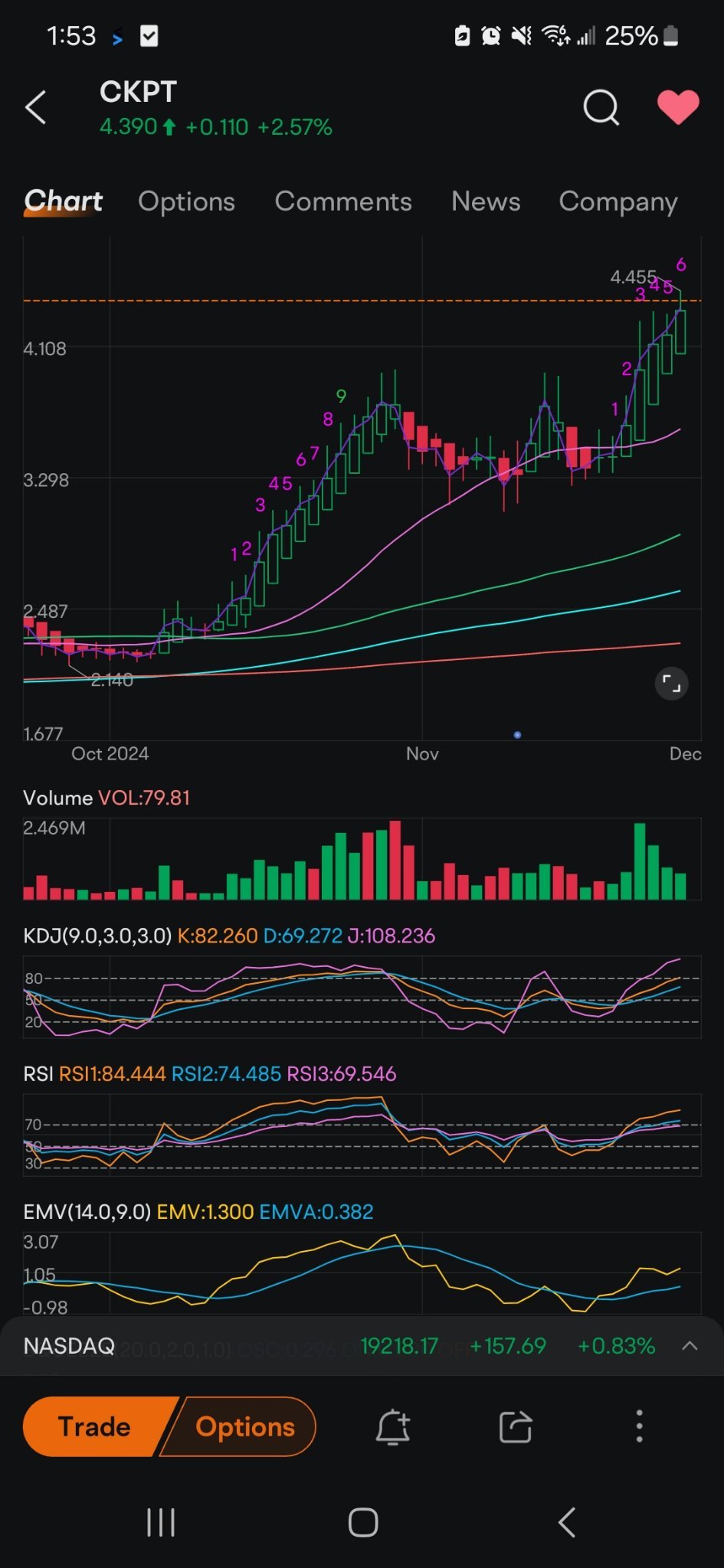
I’ve taken a position in Checkpoint Therapeutics ($CKPT) because I see strong potential in their lead drug, Cosibelimab, which is being developed to treat advanced cutaneous squamous cell carcinoma (cSCC). The clinical data has been impressive, and the FDA is set to make a decision by December 28, 2024.
The main reason I’m leaning toward approval is that the complete response letter (CRL) issued last December was only due to third-party manufacturing issues, not any concerns with the drug itself. The FDA did not raise any red flags about Cosibelimab’s safety or efficacy, so with the manufacturing issues being resolved, I’m optimistic about the drug’s approval potential.
There’s also a significant market opportunity for Cosibelimab if it gets approved, given the lack of effective treatments for cSCC. With Checkpoint’s market cap still under $250M, there’s a lot of room for growth, especially with a successful FDA approval.
I’m invested and watching CKPT closely, and I think the upside here is substantial if all goes well. Key point here is: IF ALL GOES WELL WITH THE FDA'S DECISION.