Eli Lilly's Donanemab Nears Approval Decision, Could This Be a Turning Point in Alzheimer's Treatment?
The pharma giant $Eli Lilly and Co (LLY.US)$ is on the cusp of a significant breakthrough with its Alzheimer's drug, donanemab. Following the completion of its Phase 3 trials, donanemab is currently undergoing FDA review and has the potential to receive approval later this month. If approved, it would be the second anti-Aβ therapy for Alzheimer's disease to receive full clearance from the U.S. FDA.
Alzheimer's Patient Numbers Continue to Rise
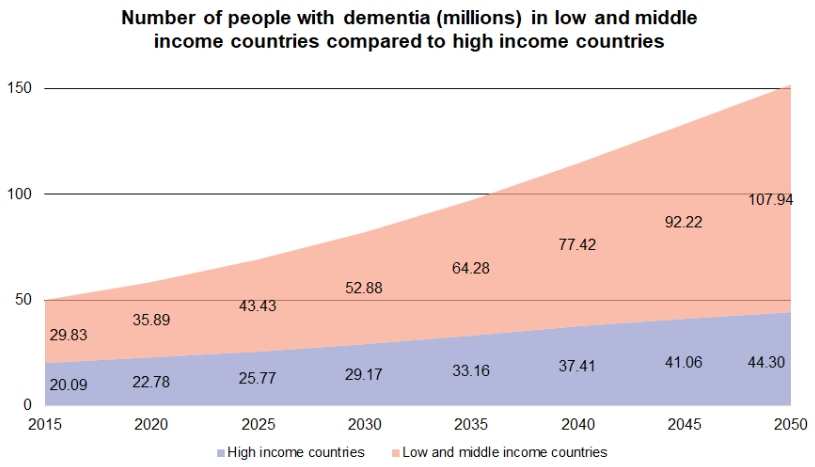
Globally, over 30 million people are grappling with the challenges of Alzheimer's disease, and this number is anticipated to rise. According to the World Health Organization, more than 55 million people currently have dementia, with Alzheimer's accounting for 60%-70% of these cases. This number is expected to rise to 82 million by 2030 and 152 million by 2050.
In response to this growing health crisis, the market for Alzheimer's drugs is also expanding. Renub Research forecasts that by 2027, the global market for Alzheimer's medications will reach an estimated $7.48 billion, propelled by a compound annual growth rate (CAGR) of 7.71% from 2022 to 2027.
What Are the Current Treatments?
Drug development for Alzheimer's disease has been a major challenge for the global pharmaceutical industry for many years. Despite the increasing number of Alzheimer's patients worldwide, progress in developing treatments for the disease has been notably slow, with a staggering 99.6% failure rate, according to articles published in Scientific American.
In recent years, as the understanding of the pathological mechanisms behind Alzheimer's disease has evolved, several new therapeutic approaches have been developed, with one of the most notable being immunotherapy targeting amyloid-beta plaques in the brain.
In June 2021, $Biogen (BIIB.US)$'s groundbreaking drug Aduhelm received FDA approval, marking the debut of the first monoclonal antibody therapy aimed at targeting amyloid-beta plaques in the brain. Yet, amidst contentious debates over its efficacy and safety, Biogen made the proactive decision to withdraw Aduhelm from the market at the start of 2023.
Leqembi, a collaborative effort from $EISAI CO LTD (ESALF.US)$ and Biogen, received full FDA approval in January 2023. Like Aduhelm, Leqembi is a monoclonal antibody targeting beta-amyloid plaques. However, it has shown more substantial clinical trial results, demonstrating a pronounced ability to slow cognitive decline in patients with early-stage Alzheimer's disease.
How Does Eli Lilly's Donanemab Stand Out?
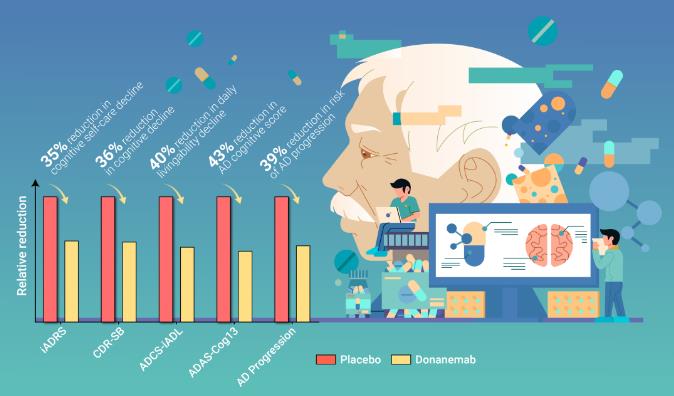
Donanemab targets a modified form of the beta-amyloid protein called N3pG, aiming to clear these plaques from the brain with precision. Last May, Eli Lilly released the latest Phase 3 clinical trial data, revealing that donanemab significantly slows cognitive and functional decline in patients with early-stage Alzheimer's disease.
In the field of Alzheimer's research, the Clinical Dementia Rating-Sum of Boxes (CDR-SB) serves as the gold standard for assessing clinical outcomes. The CDR-SB scale demonstrated that donanemab slowed the rate of cognitive decline by 36%. Notably, 47% of participants receiving donanemab experienced no progression in their CDR-SB scores over the course of a year, significantly outperforming the placebo group. In comparison, the treatment group receiving lecanemab(Leqembi) showed a 27% slower rate of cognitive decline compared to the placebo group.
Maria Carrillo, the Alzheimer's Association chief scientific officer, said in a statement,
These are the strongest phase 3 data for an Alzheimer's treatment to date. This further underscores the inflection point we are at for the Alzheimer's field.
Dr. Daniel Skovronsky, Eli Lilly chief scientific officer, is "extremely optimistic" and believes a big leap in Alzheimer's disease treatment may be just around the corner–and that an overlooked class of medicines could become the GLP-1 drugs of the future.
As we track Eli Lilly's progress with its Alzheimer's drug, it's also worth noting the role of key CDMOs like $LONZA GROUP AG UNSP ADR EACH REP 0.1 ORD (LZAGY.US)$ and $Catalent (CTLT.US)$. These globally recognized firms serve as critical partners for a myriad of pharmaceutical companies, delivering an extensive array of services that span drug development, clinical trial logistics, commercial-scale production, and packaging. Their pivotal support in drug R&D and supply chain management is an integral part of bringing therapies from the lab to the marketplace.
Source: Alzheimer's Disease International, The Innovation Medicine, CNBC, Market Watch
By Moomoo News Marina
Disclaimer: Moomoo Technologies Inc. is providing this content for information and educational use only.
Read more
Comment
Sign in to post a comment