INHALE-3 Study Reveals Positive Readout in Head-to-Head Comparison of Inhaled Insulin Vs. Usual Care in T1D; New Data Presented at American Diabetes Association's 84th Scientific Sessions
INHALE-3 Study Reveals Positive Readout in Head-to-Head Comparison of Inhaled Insulin Vs. Usual Care in T1D; New Data Presented at American Diabetes Association's 84th Scientific Sessions
- Study proves inhaled insulin is as effective as usual care (primarily automated insulin delivery pumps or multiple daily injections) for adults living with T1D meeting the primary endpoint
- Patients utilizing inhaled insulin reached target A1c (less than 7%) 30% of the time compared to 17% with usual care and 24% had time-in-range (TIR) above 70% with no increased hypoglycemia compared with 13% with usual care
- More than 50% of subjects at the end of the study expressed an interest in continuing to use Afrezza
- 研究证明,对于符合一级终点的T1D成年患者,吸入胰岛素与常规护理(主要为自动胰岛素泵或多次日常注射)的效果一样有效。
- 使用吸入胰岛素的患者在达到目标A1c(小于7%)的时间上比常规护理多30%,同时24%的患者处于时间范围内(TIR)耐受性高于70%,并且与常规护理相比没有增加低血糖的发生率,后者仅有17%和13%。
- 超过50%的受试者在研究结束时表示有兴趣继续使用Afrezza。
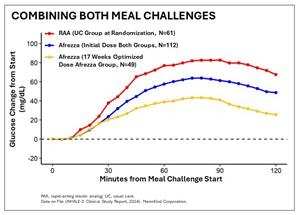
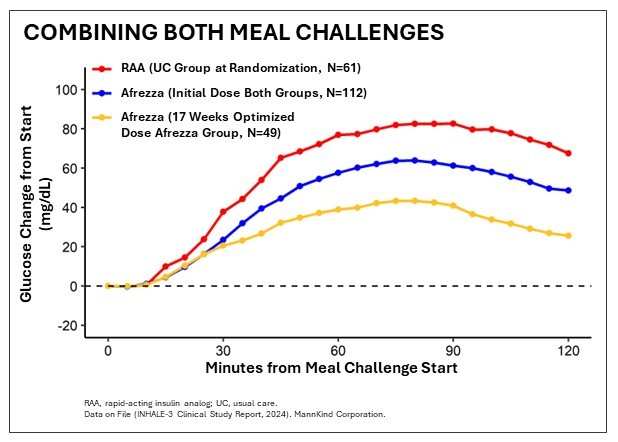
DANBURY, Conn. and WESTLAKE VILLAGE, Calif., June 22, 2024 (GLOBE NEWSWIRE) -- MannKind Corporation (Nasdaq: MNKD), a company focused on the development and commercialization of inhaled therapeutic products and devices for patients with endocrine and orphan lung diseases, today announced positive 17-week results from the INHALE-3 study, a Phase 4 U.S. clinical trial evaluating Afrezza (plus basal insulin) vs. usual care (defined as multiple daily injections (MDI), an automated insulin delivery system, (AID) or a pump without automation) utilizing a higher initial conversion dose from mealtime injectable insulin to inhaled insulin. The study, which was presented by the INHALE-3 investigational team at the American Diabetes Association's (ADA) 84th Scientific Sessions in Orlando, met its primary efficacy endpoint of a non-inferior change in HbA1c between baseline and week 17 compared to the usual care group.
2024年6月22日,康州丹伯里和加州Westlake Village — 曼恩凯德生物医疗公司(纳斯达克股票代码:根据标准板MNKD)今天宣布了INHALE-3研究的积极17周结果,INHALE-3研究是一项第4阶段的美国临床试验,评估吸入式治疗产品和设备对内分泌和孤儿(罕见)肺病患者的疗效。(加基础胰岛素)与普通护理相比,使用吸入胰岛素将膳食注射胰岛素的初始转换剂量提高。该研究由INHALE-3研究小组在奥兰多召开的美国糖尿病协会(ADA)的84项科学会议上发表,并实现了其主要疗效终点。th(基础胰岛素)与普通护理(包括AID泵)相结合时,吸入胰岛素对HbA1c / TIR的效应类似于常规护理,而没有新的安全问题。
Key sub-analysis findings included:
重要的分析结果包括:
- More subjects utilizing inhaled insulin achieved glycemic targets:
- 30% of inhaled insulin group reached <7% (HbA1c) at 17 weeks vs. 17% of the usual care group
- 21% of inhaled insulin group vs. 0% of usual care group met A1c goal of <7% if baseline was >7%
- 24% of the Afrezza group and 13% of the usual care group achieved TIR above 70% with no increased hypoglycemia in the inhaled insulin group
- No difference in CGM-measured hypoglycemia between the groups
- Study helps to establish a titrated basal-bolus ratio that is approximately 70/30 inhaled insulin to basal vs. 50/50 for usual care
- While more people met the glycemic target of A1c (less than 7%) with Afrezza, some subjects worsened when switching from usual care to inhaled insulin, potentially due to missing doses of inhaled insulin during the day and/or underdosing going into bedtime
- More than 50% of subjects at the end of the study expressed an interest in continuing to use Afrezza
- 更多使用吸入式胰岛素的对象达到了血糖控制目标:
- 吸入胰岛素组在17周时达到HbA1c<7%的患者占30%,而常规护理组仅为17%。
- 吸入胰岛素组和常规护理组之间,如果基线为>7%,约有21%的吸入胰岛素组和0%的常规护理组达到A1c目标<7%。
- 24%的Afrezza组和13%的常规护理组在吸入胰岛素组中实现了TIR高于70%,而没有增加低血糖的发生率。
- 两组之间CGM测量的低血糖没有差异。
- 研究有助于确定调整后的基础/远程注射比约为70/30的吸入胰岛素比例与常规护理的50/50相比。
- 虽然更多的人在使用Afrezza后达到了A1c(小于7%)的糖化目标,但一些受试者在从常规护理转换到吸入胰岛素时病情加剧,可能是因为错过了一些白天吸入胰岛素的剂量和/或晚上没吸入足够剂量。
- 超过50%的受试者在研究结束时表示有兴趣继续使用Afrezza。
"Inhaled insulin demonstrated improved mealtime control, which is significant given how this continues to be a significant unmet need," said Dr. Irl Hirsch, Professor of Medicine and Diabetes Treatment and Teaching Chair at the University of Washington and the INHALE-3 Study Protocol Chair. "The INHALE-3 study delivered data that supports inhaled insulin being an important treatment option for adults living with diabetes."
“吸入胰岛素证明了改善膳食控制的效果,这是因为这仍然是一个重要的未满足的需求,”因华盛顿大学的内分泌和糖尿病治疗与教学主席和INHALE-3研究协议主席Irl Hirsch博士说:“INHALE -3研究提供了支持吸入胰岛素作为治疗成人糖尿病患者的重要选择的数据。”
"INHALE-3 adds to the body of evidence that when combined with basal insulin, inhaled insulin's effect on HbA1c/TIR is similar to that of the usual care (inclusive of AID pumps) with no new safety concerns," said Dr. Kevin Kaiserman, Senior Vice President, Clinical Development and Medical Affairs for MannKind Corporation. "Our data continues to show the importance of Afrezza as a safe and effective tool for managing diabetes."
曼恩凯德公司临床开发和医学事务高级副总裁凯文·凯塞曼博士说:“INHALE-3研究为常规护理(包括AID泵)结合基础胰岛素时结合吸入胰岛素HbA1c / TIR的效应增加了更多的证据,并没有新的安全问题。我们的数据继续显示Afrezza作为管理糖尿病的安全有效工具的重要性。”
The INHALE-3 study is a 17-week, randomized controlled trial with a 13-week extension conducted across 19 U.S. sites. The study, which enrolled 141 patients (123 randomized), assigned participants over 18 years of age with T1D who are using MDI, an automated insulin delivery system, or a pump without automation to either continue their standard of care or initiate an insulin regimen of a daily basal injection plus Afrezza for boluses (mealtime and corrections). Both arms utilized continuous glucose monitoring to assess glucose control. A1c levels were obtained at baseline, 17 and 30-weeks. The full 30-week results of INHALE-3 will be presented at future conferences. More information on the INHALE-3 study is available at: ClinicalTrials.gov(NCT05904743).
INHALE-3研究是一项为期17周的随机对照试验,进行于美国19个试点机构。该研究招募了141名年龄在18岁以上的T1D患者,使用MDI、自动胰岛素输送系统或泵不带自动化,分配参与者继续标准疗法或开始注射每日基础胰岛素加上用于胰岛素峰值(餐后和矫正)的Afrezza。两组均使用连续血糖监测来评估血糖控制。在基线、17周和30周分别获取A1c水平。INHALE-3的完整30周结果将在未来的会议上展示。关于INHALE-3研究的更多信息可访问:ClinicalTrials.gov(NCT05904743).
About Afrezza
Afrezza (insulin human) Inhalation Powder is a rapid-acting inhaled human insulin indicated to improve glycemic control in adults with diabetes mellitus.
关于Afrezza
Afrezza(人类胰岛素)吸入粉末是一种快速作用的吸入式人类胰岛素,可改善成人糖尿病患者的血糖控制。
Limitations of Use: Not recommended for the treatment of diabetic ketoacidosis or in patients that smoke or have recently stopped smoking.
使用该药物不推荐治疗糖尿病酮症或吸烟或最近戒烟的患者。
Important Safety Information
重要安全信息
WARNING: RISK OF ACUTE BRONCHOSPASM IN PATIENTS WITH CHRONIC LUNG DISEASE
警告:对于患有慢性肺部疾病的患者有急性支气管痉挛的风险。
- Acute bronchospasm has been observed in Afrezza-treated patients with asthma and COPD
- Afrezza is contraindicated in patients with chronic lung disease such as asthma or COPD
- Before initiating Afrezza, perform a detailed medical history, physical examination, and spirometry (FEV1) to identify potential lung disease in all patients.
- 曾观察到Afrezza治疗的哮喘和COPD患者出现急性支气管痉挛。
- Afrezza在慢性肺病如哮喘或COPD患者中是禁忌的。
- 在开始使用Afrezza之前,对所有患者进行详细的病史、体检和肺功能检查(FEV)。1最常见的副作用是低血糖、咳嗽和喉咙疼痛或刺激。
Most common adverse reactions are hypoglycemia, cough, and throat pain or irritation.
请在Afrezza.com/safety上查看其他重要安全信息、全面处方信息,包括加粗警告。
Please see additional Important Safety Information, Full Prescribing Information, including BOXED WARNING, available on Afrezza.com/safety.
请查阅附加的重要安全信息、完整处方信息(包括加框警告),网址为:Afrezza.com/safety.
About MannKind
MannKind Corporation (Nasdaq: MNKD) focuses on the development and commercialization of innovative inhaled therapeutic products and devices to address serious unmet medical needs for those living with endocrine and orphan lung diseases.
曼恩凯德生物医疗公司(Nasdaq:MNKD)专注于为那些患有内分泌和孤儿肺病的患者开发和商业化创新吸入治疗产品和设备。
我们致力于利用我们的配方能力和设备工程的技术,减轻糖尿病,非结核分枝杆菌(NTM)肺病,肺纤维化和肺动脉高压等疾病的负担。我们的标志性技术——干粉配方和吸入设备——可以快速,方便地向肺的深处输送药物,从而在目标疾病的治疗方面产生局部或进入系统循环的作用,具体取决于治疗目标。
We are committed to using our formulation capabilities and device engineering prowess to lessen the burden of diseases such as diabetes, nontuberculous mycobacterial (NTM) lung disease, pulmonary fibrosis, and pulmonary hypertension. Our signature technologies – dry-powder formulations and inhalation devices – offer rapid and convenient delivery of medicines to the deep lung where they can exert an effect locally or enter the systemic circulation, depending on the target indication.
在整个团队以及全国范围内合作的曼尼海特人的激情下,我们致力于实现让人们控制自己的健康和自由自在地生活的使命。
With a passionate team of Mannitarians collaborating nationwide, we are on a mission to give people control of their health and the freedom to live life.
mannkindcorp.com了解更多信息,以及关注我们的消息。
Please visit mannkindcorp.com to learn more, and follow us on LinkedIn, Facebook, X or Instagram.
请访问mannkindcorp.com了解更多信息,关注我们。LinkedIn, Facebook, X或。Instagram.
Forward-Looking Statements
This press release contains forward-looking statements about the planned release of results from an ongoing clinical study that involves risks and uncertainties. Words such as "believes", "anticipates", "plans", "expects", "intends", "will", "goal", "potential" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon MannKind's current expectations. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the risk that we may not achieve our projected development goals in the timeframes we expect, the risk that continued testing of our products may not yield successful results as well as other risks detailed in MannKind's filings with the Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2023, and subsequent periodic reports on Form 10-Q and current reports on Form 8-K. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. All forward-looking statements are qualified in their entirety by this cautionary statement, and MannKind undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date of this press release.
前瞻性声明
本新闻发布涉及正在进行的临床研究计划结果的前瞻性声明,存在风险和不确定性。"相信","预计","计划","期望","打算","将","目标","潜在"和类似表达意图的词语,旨在识别前瞻性声明。这些前瞻性声明基于曼恩凯德生物医疗目前的期望。事实结果和事件的时间可能因各种风险和不确定性而与上述前瞻性声明有所不同,这些风险和不确定性包括但不限于,我们可能无法按照预期时间框架实现预测的发展目标的风险,我们的产品持续测试可能不会产生成功的结果以及其他在曼恩凯德生物医疗提交给证券交易委员会的备案中详细说明的风险,包括其截至2023年12月31日的年度报告表格10-K,以及随后的周期性报告表格10-Q和目前的报告表格8-K。请注意不要过度依赖这些前瞻性声明,这些前瞻性声明仅适用于本新闻发布日。所有前瞻性声明均由此警示语句限定其全部内容,曼恩凯德生物医疗不承担任何更新或修订任何前瞻性声明以反映本新闻发布日后的事件或情况的义务。
AFREZZA and MANNKIND are registered trademarks of MannKind Corporation.
AFREZZA和曼恩凯德均为MannKind Corporation的注册商标。
For MannKind:
对于曼恩凯德生物医疗:
Christie Iacangelo, Corporate Communications
(818) 292-3500
Email: media@mannkindcorp.com
Christie Iacangelo,企业传播
(818)292-3500
电子邮件: media@mannkindcorp.com
Photos accompanying this announcement are available at:
附带此公告的照片可在以下链接中找到:
Source: MannKind
来源:曼恩凯德生物医疗