Market Mover | Vaxcyte Shares Surge 36% After Positive Topline Data from 1/2 Study of VAX-31 Vaccine
Market Mover | Vaxcyte Shares Surge 36% After Positive Topline Data from 1/2 Study of VAX-31 Vaccine
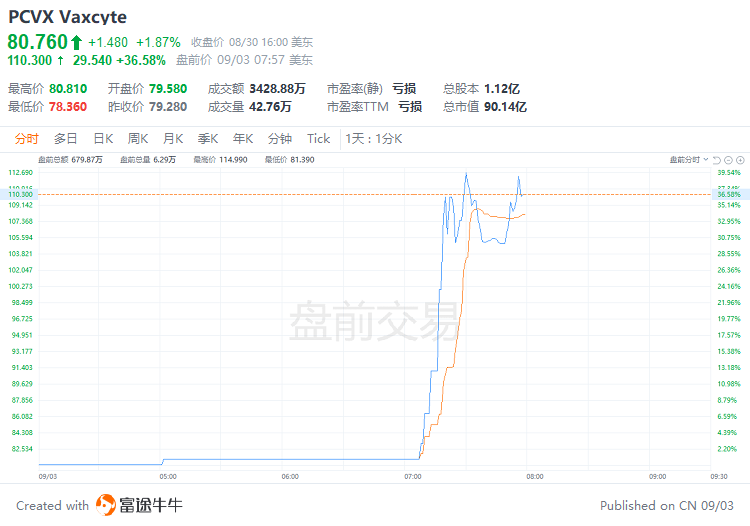
September 3, 2024 - $Vaxcyte (PCVX.US)$shares surged 36.58% to $110.30 in pre-market trading on Tuesday. The company today announced positive topline results from the Phase 1/2 study evaluating the safety, tolerability and immunogenicity of VAX-31, the Company’s 31-valent pneumococcal conjugate vaccine (PCV) candidate designed to prevent invasive pneumococcal disease (IPD), in 1,015 healthy adults aged 50 and older.
2024年9月3日- $Vaxcyte (PCVX.US)$股票在周二美股盘前交易中上涨36.58%,达到110.30美元。该公司今天宣布了Phase 1/2研究的积极顶线结果,评估了VAX-31的安全性、耐受性和免疫原性,该疫苗是该公司的31价肺炎球菌结合疫苗(PCV)候选药物,旨在预防侵袭性肺炎球菌感染(IPD),在1015名50岁及以上健康成年人中进行。
VAX-31 generated strong immune responses for all 31 strains of pneumococcus it targets. For the 20 strains it shares with Prevnar 20, VAX-31 met or exceeded immune response criteria at medium and high doses. For the additional 11 strains unique to VAX-31, it met all superiority criteria at all doses.
VAX-31针对31个肺炎球菌菌株产生了强有效的免疫反应。对于与Prevnar 20共有的20个菌株,VAX-31在中、高剂量下达到或超过了免疫反应标准。对于VAX-31独有的11个菌株,它在所有剂量下均满足了所有优势标准。
Based on these promising results, Vaxcyte plans to move VAX-31 to a larger Phase 3 study by mid-2025, with results expected in 2026. The company aims to provide broader protection against pneumococcal disease in adults over 50, addressing an important public health need.
基于这些令人鼓舞的结果,Vaxcyte计划在2025年中期将VAX-31进入更大规模的Phase 3研究,并预计在2026年公布结果。该公司旨在为50岁以上的成年人提供更广泛的肺炎球菌疾病保护,满足重要的公共健康需求。
About Vaxcyte
关于Vaxcyte
Vaxcyte is a vaccine innovation company engineering high-fidelity vaccines to protect humankind from the consequences of bacterial diseases. The Company is developing broad-spectrum conjugate and novel protein vaccines to prevent or treat bacterial infectious diseases. VAX-31 is a Phase 3-ready 31-valent, carrier-sparing PCV being developed for the prevention of IPD in adults and infants and is the broadest-spectrum PCV candidate in the clinic today. VAX-24, the Company’s 24-valent PCV candidate, is designed to cover more serotypes than any infant PCV on-market and is currently being evaluated in a Phase 2 infant study. Both VAX-31 and VAX-24 are designed to improve upon the standard-of-care PCVs by covering the serotypes in circulation that are responsible for a significant portion of IPD and are associated with high case-fatality rates, antibiotic resistance and meningitis, while maintaining coverage of previously circulating strains that are currently contained through continued vaccination practice.
Vaxcyte是一家致力于疫苗创新的公司,致力于开发高保真疫苗以保护人类免受细菌性疾病的影响。该公司正在研发广谱结合疫苗和新型蛋白疫苗,以预防或治疗细菌感染性疾病。VAX-31是一种准备进入Phase 3试验的31价,无载体PCV,专为预防成年人和婴儿的IPD,是目前临床上最广谱的PCV候选药物。VAX-24是该公司的24价PCV候选药物,设计覆盖了市场上任何婴儿PCV的更多血清型别,并且目前正在进行Phase 2婴儿研究。VAX-31和VAX-24都旨在改进目前的标准护理PCV,覆盖目前循环的那些导致重要部分IPD、耐药性和脑膜炎的血清型别,同时保持对以前循环的菌株的覆盖,这些菌株目前通过继续接种实践持续控制。
Related Reading: Press Release
相关阅读:新闻发布
