Biohaven Announces 3-Year Results for Troriluzole in Spinocerebellar Ataxia Treatment
Biohaven Announces 3-Year Results for Troriluzole in Spinocerebellar Ataxia Treatment
Biohaven Ltd. (NYSE:BHVN) (Biohaven or the Company), today announced positive topline results from pivotal Study BHV4157-206-RWE (NCT06529146) demonstrating the efficacy of troriluzole on the mean change from baseline in the f-SARA after 3 years of treatment. The study achieved the primary endpoint and showed statistically significant improvements on the f-SARA at years 1 and 2 (Figure 1). SCA is a rare, progressively debilitating neurodegenerative disease that affects approximately 15,000 people in the United States and 24,000 in Europe and the United Kingdom. There are no FDA approved treatments for SCA.
Biohaven有限公司(纽交所:BHVN)(Biohaven或公司)今天宣布关于关键研究BHV4157-206-RWE(NCT06529146)的高端结果,显示troriluzole对经过3年治疗后f-SARA基线平均变化的疗效。该研究实现了主要终点,并表现出在第1年和第2年在f-SARA上有显著改善(图1)。SCA是一种罕见的、日益恶化的神经退行性疾病,影响美国约15,000人,欧洲和英国约24,000人。目前还没有FDA批准的SCA治疗方法。
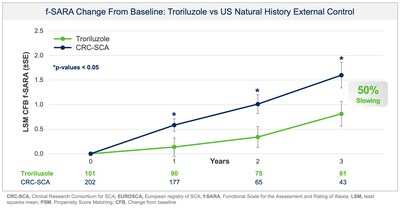
Collectively, data across multiple analyses demonstrate a robust and clinically meaningful slowing of disease progression in SCA patients. These treatment benefits translate into a 50-70% slower rate of decline compared to untreated patients, representing 1.5-2.2 years delay in disease progression over the 3-year study period. Additionally, in a responder sensitivity analysis, disease progression when defined by a 2 point or greater worsening on the f-SARA at 3 years showed an odds ratio (OR) of 4.1 (95% CI: 2.1, 8.1) for the untreated external control arm versus troriluzole treated subjects (p < 0.0001; pooled analysis).
跨多个分析的数据合集显示,对SCA患者疾病进展减缓具有强大且临床意义重大的效果。这些治疗效益转化为疾病进展速度比未治疗患者慢50-70%,相当于在3年研究期间延迟疾病进展1.5-2.2年。此外,在一个反应敏感性分析中,根据f-SARA在3年内定义的2分或更大恶化来定义的疾病进展显示,未经troriluzole治疗的外部对照组与接受troriluzole治疗的受试者(p
Dr. Susan Perlman, Director of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA stated, "SCA is a debilitating, relentlessly progressive disease that destroys quality of life, leaving patients unable to care for themselves, walk, or speak. Troriluzole is the very first treatment to show a delay in disease progression that can give patients additional years of independence, where they can walk without assistance, continue to work, play with their children, and participate in daily activities. This is an exciting and hopeful moment for the SCA community."
加州大学洛杉矶分校大卫盖芬医学院共济失调诊所和神经遗传学临床试验主任苏珊·珀尔曼博士表示:“SCA是一种毁灭生活质量、不断进展的疾病,导致患者无法自理、行走或讲话。Troriluzole是第一个显示能延缓疾病进展的治疗方法,可以使患者增加独立生活的岁月,能够在无需帮助的情况下行走,继续工作,与孩子玩耍,并参与日常活动。这对SCA社区来说是一个振奋人心且充满希望的时刻。”
Study BHV4157-206-RWE was designed, in discussion with the US Food and Drug Administration (FDA), to assess the effectiveness of troriluzole in SCA after 3 years of treatment as measured by the change from baseline in the f-SARA. The study utilized Phase 3 data and an external control of matched, untreated SCA subjects from the US Clinical Research Consortium for the Study of Cerebellar Ataxia (CRC-SCA) in accordance with FDA's Guidance on Real-World Evidence (RWE) of effectiveness. All endpoints were prespecified, and both the study protocol and statistical analysis plan were submitted to, and reviewed by, FDA prior to topline data analysis. The new analysis doubled the previously available 3 year data with 63 subjects now completing 3 years of treatment with troriluzole and matched to the external control arm. Propensity Score Matching (PSM) was used to ensure that untreated patients from the CRC-SCA study were rigorously matched to treated patients from Study BHV4157-206 on baseline characteristics. The primary objective was to examine the treatment effects of troriluzole for up to 3 years, by comparing data on the f-SARA from patients treated with troriluzole in Study BHV4157-206 to untreated patients from the natural history study. Troriluzole-treated patients demonstrated statistically significant and sustained benefits at years 1, 2 and 3 on the f-SARA compared to a rigorously matched natural history control.
BHV4157-206-RWE研究经过与美国食品药品监督管理局(FDA)讨论,旨在评估troriluzole在SCA患者身上的疗效,经过3年治疗后,以f-SARA基线变化为指标。该研究利用了3期数据,并在FDA的《有关真实世界证据(RWE)的有效性指导意见》的指导下,使用了来自美国小脑共济失调研究质询局(CRC-SCA)的匹配的未治疗SCA受试者的外部对照组。所有终点在预先设定,并在报告终端数据分析之前向FDA提交和评审了研究方案和统计分析计划。新的分析使之前可用的3年数据翻了一番,目前有63名受试者完成了3年的troriluzole治疗,与外部对照组匹配。使用倾向评分匹配(PSM)确保了CRC-SCA研究中未经治疗的患者与BHV4157-206研究中治疗患者在基线特征上严格匹配。主要目标是通过比较BHV4157-206研究中接受troriluzole治疗的患者与自然病程研究中未经治疗的患者的f-SARA数据,检验troriluzole的治疗效果长达3年。与从自然史研究中严格匹配的未经治疗患者相比,接受troriluzole治疗的患者在年度1、2和3的f-SARA上表现出了显著且持续的益处。
Additionally, prespecified analyses in the protocol employed a separate, independent natural history control from the European SCA natural history study (EUROSCA) for global regulatory purposes. Results using the EUROSCA patients, in addition to a pooled analysis using both CRC-SCA and EUROSCA patients, as the external controls were also statistically significant and consistent with the primary efficacy analysis at all timepoints (see Figure 2 and Figure 3). The addition of EUROSCA data increased the external control sample size and added to the robustness of the statistically significant treatment differences at years 1, 2, and 3, favoring troriluzole.
此外,协议中的预定分析采用了欧洲自然病史研究(EUROSCA)中的独立自然病史对照,用于全球监管目的。在使用EUROSCA患者的结果中,以及在使用CRC-SCA和EUROSCA患者进行汇总分析作为外部对照时,所有时间点的结果均显著且与一线功效分析一致(见图2和图3)。增加EUROSCA数据有助于提升外部对照样本量,并增加了在第1、2和3年时显著的治疗差异的稳固性,有利于troriluzole。
Jeremy Schmahmann, M.D., Professor of Neurology at Harvard Medical School and Founding Director of the Ataxia Center at Massachusetts General Hospital commented, "The stabilization of SCA symptoms as reflected by the topline data at 3 years along with the previously reported reductions in falls show the therapeutic potential of troriluzole. I cannot underscore enough the impact of a potential treatment that can slow SCA disease progression and the effect on patients and caregivers who have helplessly watched generations of family members deteriorate and die from SCA. These new data provide support for troriluzole as a safe and effective once daily treatment for patients with SCA."
哈佛医学院神经病学教授、马萨诸塞州总医院共济中心创始董事Jeremy Schmahmann万.D.评论道:“三年内一线数据反映出的SCA症状稳定,以及先前报道的摔倒减少显示了troriluzole的治疗潜力。我无法强调潜在能够减缓SCA疾病进展并影响曾经无助地看着家族成员一代代从SCA恶化并死去的患者和护理人员的治疗的影响。这些新数据支持troriluzole作为SCA患者安全有效的一日一次治疗。”
Spinocerebellar ataxia is a group of dominantly inherited neurodegenerative disorders characterized by progressive loss of voluntary motor control and atrophy of the cerebellum, brainstem and spinal cord. Patients experience significant morbidity, including progression to a wheelchair, impaired gait leading to falls, inability to communicate due to speech impairment, difficulty swallowing, and premature death. While signs and symptoms can appear anytime from childhood to late adulthood, SCA typically presents in early adulthood and progresses over a number of years. Currently, there are no FDA-approved treatments and no cure for SCA.
脊髓小脑共济失调是一组显性遗传的神经退行性障碍,其特点是逐渐失去自主运动控制和小脑、脑干和脊髓萎缩。患者会经历显著的发病率,包括进展到坐轮椅、步行困难导致摔倒、由于言语障碍无法交流、吞咽困难以及过早死亡等情况。虽然症状可以在任何时候从儿童到晚年出现,但SCA通常在早成年出现并在多年内加剧。目前,SCA没有FDA批准的治疗方法,也没有治愈方案。
Vlad Coric, M.D., Chief Executive Officer and Chairman of Biohaven stated, "Advancing new therapies for patients with rare diseases is often a multiyear process of collaboration across academic, patient advocacy, regulatory and industry partners. The Biohaven team has always been committed to rigorously following the science in this area, and through our partnership with the National Ataxia Foundation and collaboration with leading SCA experts across the globe, our SCA development program has provided the first evidence of a clinically meaningful treatment benefit as well as slowing disease progression in SCA patients. We were excited to receive the positive topline results from Study BHV4157-206-RWE, which was designed with FDA input and pursuant to the principles outlined in the FDA's guidance for the use of real-world evidence. The need for treatments for this deadly neurodegenerative disease is urgent. As a company, we remain committed to developing novel therapies for patients living with rare disorders with no approved therapies, like SCA. We look forward to interacting with regulatory agencies to bring troriluzole to patients with SCA."
biohaven的首席执行官兼主席Vlad Coric万.D.表示:“为罕见病患者推进新疗法往往是一个跨学术、患者倡导、监管和行业合作的多年过程。Biohaven团队始终致力于严格遵循这一领域的科学,通过与全球领先的SCA专家合作,我们的SCA开发计划首次提供了临床上有意义的治疗益处的证据,并减缓了SCA患者的疾病进展。我们对收到的研究BHV4157-206-RWE的积极一线结果感到激动,该研究是在FDA的指导以及遵照FDA关于使用真实世界证据的准则进行,并获得了FDA的支持。治疗这种致命神经退行性疾病的需求紧迫。作为一家公司,我们致力于为患有没有批准疗法的罕见疾病的患者开发新疗法,如SCA。我们期待与监管机构互动,将troriluzole带给SCA患者。”
Andrew Rosen, Chief Executive Officer of the National Ataxia Foundation (NAF), shared, "Biohaven was the first company to join NAF's Drug Development Collaborative (DDC), a group of pharmaceutical companies dedicated to bringing together advocates, clinicians, regulatory agencies, and the patient community to advance research and facilitate the development of therapies for ataxia. Today's topline results are the culmination of years dedicated to studying troriluzole as a treatment for SCA. Patients and families have been waiting for decades for a treatment that could slow disease progression in this devastating and relentlessly progressive disorder".
Andrew Rosen,国家共济会(NAF)首席执行官分享道:"biohaven是第一家加入NAF药物开发合作伙伴关系(DDC)的公司,这是一群致力于汇集倡导者、临床医生、监管机构和患者社区的制药公司,旨在推动研究并促进共济会治疗疗法的发展。今天的最新结果是多年研究troriluzole作为SCA治疗手段的最终成果。患者和家庭已经等待数十年,期待一种可以减缓这种毁灭性和不可抗拒的进行性疾病病情恶化的治疗"。
Based upon the topline data from Study BHV4157-206-RWE, and previous safety and efficacy data from the troriluzole development program in SCA, Biohaven plans to submit a New Drug Application (NDA) to the FDA in Q4 2024. The troriluzole development program has generated the largest clinical trial dataset in SCA and now has follow-up in some patients treated with troriluzole for over 5 years. Biohaven has previously received both Fast-Track and Orphan drug designation (ODD) from the FDA, and ODD from the European Medicines Agency, for troriluzole in SCA. An NDA with ODD is eligible for priority FDA review. Biohaven will be prepared to commercialize SCA in the US in 2025, if ultimately approved, based on potential priority review timelines.
根据研究BHV4157-206-RWE的最新数据,以及SCA中troriluzole开发项目中之前的安全性和有效性数据,biohaven计划在2024年第四季度向美国食品药品监督管理局提交新药申请(NDA)。 troriluzole开发项目已经产生了SCA中最大规模的临床试验数据,并且现在在一些接受troriluzole治疗超过5年的患者中进行了后续随访。Biohaven此前已从FDA获得了快速通道和孤儿药品认定(ODD),并从欧洲药品管理局获得了ODD,用于SCA中的troriluzole。获得ODD的NDA有资格优先获得FDA审查。如果最终获得批准,biohaven将准备在2025年在美国商业化SCA,根据可能的优先审查时间表。

 Dr. Susan Perlman, Director of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA stated, "SCA is a debilitating, relentlessly progressive disease that destroys quality of life, leaving patients unable to care for themselves, walk, or speak. Troriluzole is the very first treatment to show a delay in disease progression that can give patients additional years of independence, where they can walk without assistance, continue to work, play with their children, and participate in daily activities. This is an exciting and hopeful moment for the SCA community."
Dr. Susan Perlman, Director of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA stated, "SCA is a debilitating, relentlessly progressive disease that destroys quality of life, leaving patients unable to care for themselves, walk, or speak. Troriluzole is the very first treatment to show a delay in disease progression that can give patients additional years of independence, where they can walk without assistance, continue to work, play with their children, and participate in daily activities. This is an exciting and hopeful moment for the SCA community."