MannKind Shares Are Falling Today: What's Going On?
MannKind Shares Are Falling Today: What's Going On?
MannKind Corporation (NASDAQ:MNKD) are trading lower Monday. The company released its six-month results from its Phase 3 INHALE-1 pediatric diabetes trial. Here's what you need to know.
周一,曼肯德公司(纳斯达克股票代码:MNKD)股价走低。该公司公布了其三期 INHALE-1 儿科糖尿病试验的六个月结果。以下是你需要知道的。
What To Know: The trial investigated the use of Afrezza (insulin human) Inhalation Powder in children and adolescents aged 4 to 17 years. Its primary endpoint was a non-inferior change in HbA1c levels after 26 weeks of treatment.
须知:该试验调查了Afrezza(人胰岛素)吸入粉末在4至17岁儿童和青少年中的使用情况。其主要终点是治疗26周后HbA1c水平的变化不逊色。
The results showed that, while Afrezza met the non-inferiority criteria based on a modified intent-to-treat (mITT) analysis, a full intent-to-treat (ITT) analysis did not meet the predetermined non-inferiority margin, largely due to the variability introduced by one non-compliant patient. Despite this, the mITT analysis supported Afrezza's non-inferiority to multiple daily injections (MDI) of rapid-acting insulin.
结果显示,尽管根据修改后的意向治疗(MiTT)分析,Afrezza符合非劣势标准,但全面的意向治疗(ITT)分析并未达到预先确定的非劣势范围,这主要是由于一名不合规患者引入了变异性。尽管如此,MiTT的分析支持Afrezza并不逊色于每日多次注射速效胰岛素(MDI)。
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
该研究还显示,Afrezza和MDI组之间的肺功能没有显著差异,两个治疗组在26周内保持稳定的FEV1水平。两种治疗方案之间没有发现任何重大的安全问题,包括低血糖。
Despite the encouraging efficacy and safety outcomes, the stock's decline reflects investor cautiousness, as the company now plans to meet with the U.S. Food and Drug Administration (FDA) in the first half of 2025 regarding a supplemental new drug application (sNDA) for Afrezza's use in pediatric populations. This meeting is crucial for the potential next steps in expanding the treatment's approval, which may contribute to further volatility in the stock price depending on the regulatory response.
尽管疗效和安全结果令人鼓舞,但该股的下跌反映了投资者的谨慎态度,因为该公司现在计划在2025年上半年与美国食品药品监督管理局(FDA)会面,讨论Afrezza在儿科人群中使用的补充新药申请(snDa)。这次会议对于在扩大该疗法批准范围方面可能采取的下一步措施至关重要,这可能会导致股价进一步波动,具体取决于监管部门的应对措施。
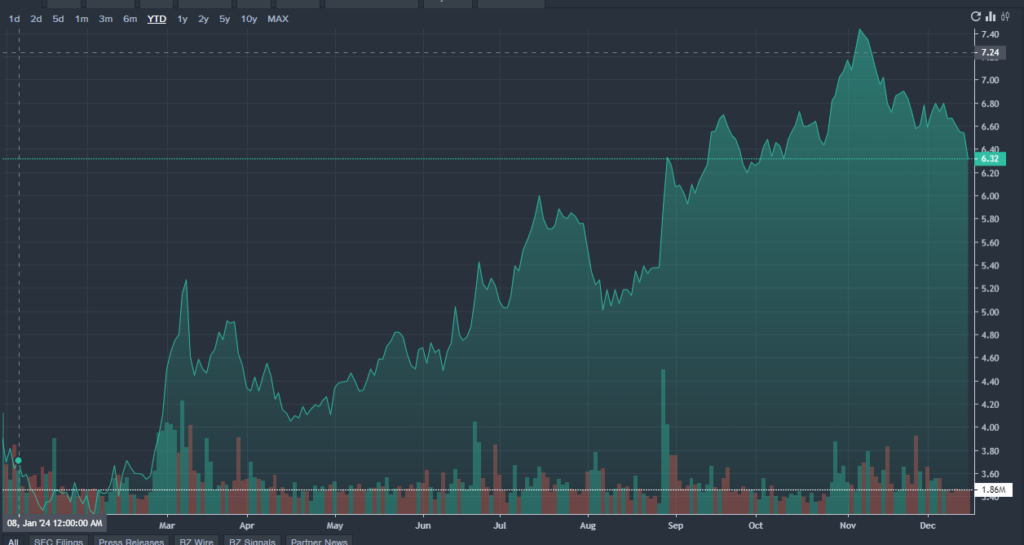
MNKD Price Action: MannKind shares were down 4.28% at $6.27 at the time of writing, according to Benzinga Pro.
MNKD价格走势:根据Benzinga Pro的数据,在撰写本文时,MannKind股价下跌4.28%,至6.27美元。
- Nvidia And 5 Other Stocks Are Analyst's Top Semiconductor Picks For 2025, Sees 2 AI Trends
- 英伟达和其他5只股票是分析师2025年的首选半导体,预计有2种人工智能趋势
Image Via Shutterstock.
图片来自 Shutterstock。

 The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.