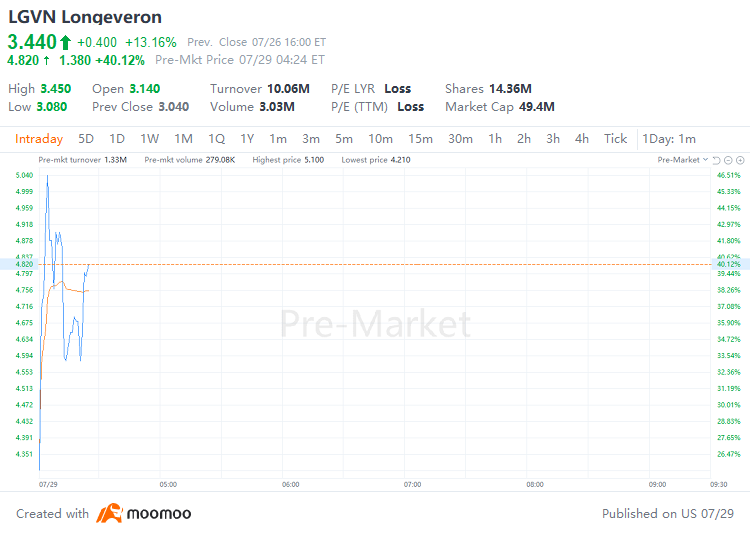
July 29, 2024 - $Longeveron (LGVN.US)$shares surged 40.12% to $4.82 in pre-market trading on Monday. The company has reported positive findings from its Phase 2a CLEAR MIND clinical trial at the Alzheimer’s Association International Conference in Philadelphia.
Longeveron's product, Lomecel-B™️, is a cell therapy aimed at treating Alzheimer's Disease by reducing brain inflammation and slowing the disease's progression. The trial, which involved 48 older adults with mild Alzheimer's, met its primary safety goals and showed promising efficacy in slowing cognitive and functional decline. Lomecel-B™️ was well-tolerated with no serious side effects and was also associated with a reduction in brain volume loss and improvements in daily living activities and quality of life for patients, as observed by their caregivers. These results suggest Lomecel-B™️ may be a valuable future treatment for Alzheimer's Disease.
“In recent years, we’ve seen an enhanced industry focus on bringing novel Alzheimer’s Disease therapeutics to market to treat the millions of people who suffer each year,” said Nataliya Agafonova, M.D., Chief Medical Officer at Longeveron. “The emergence of new Alzheimer’s Disease therapies has demonstrated that we are increasingly capable of treating a condition once deemed untreatable, and the next step in this endeavor is to develop a therapeutic that is both safe and effective in treating this disease. The results from the Phase 2a CLEAR MIND trial are highly encouraging and demonstrate the potential of Lomecel-B™️ to fulfill this need, and I look forward to its continued development.”
About Lomecel-B™️
 Lomecel-B™️ is a living cell product made from specialized cells isolated from the bone marrow of young healthy adult donors. These specialized cells, known as medicinal signaling cells (MSCs), are essential to our endogenous biological repair mechanism. MSCs have been shown to perform a number of complex functions in the body, including the formation of new tissue. They also have been shown to respond to sites of injury or disease and secrete bioactive factors that are immunomodulatory and regenerative. We believe that Lomecel-B™️ may have multiple potential mechanisms of action that may lead to anti-inflammatory, pro-vascular regenerative responses, and therefore may have broad application for a range of rare and aging related diseases.
Lomecel-B™️ is a living cell product made from specialized cells isolated from the bone marrow of young healthy adult donors. These specialized cells, known as medicinal signaling cells (MSCs), are essential to our endogenous biological repair mechanism. MSCs have been shown to perform a number of complex functions in the body, including the formation of new tissue. They also have been shown to respond to sites of injury or disease and secrete bioactive factors that are immunomodulatory and regenerative. We believe that Lomecel-B™️ may have multiple potential mechanisms of action that may lead to anti-inflammatory, pro-vascular regenerative responses, and therefore may have broad application for a range of rare and aging related diseases.
About Longeveron Inc.
Longeveron is a clinical stage biotechnology company developing regenerative medicines to address unmet medical needs. The Company’s lead investigational product is Lomecel-B™️, an allogeneic medicinal signaling cell (MSC) therapy product isolated from the bone marrow of young, healthy adult donors. Lomecel-B™️ has multiple potential mechanisms of action encompassing pro-vascular, pro-regenerative, anti-inflammatory, and tissue repair and healing effects with broad potential applications across a spectrum of disease areas. Longeveron is currently pursuing three pipeline indications: hypoplastic left heart syndrome (HLHS), Alzheimer’s Disease, and Aging-related Frailty. Lomecel-BTM development programs have received five separate and distinct FDA designations: for the HLHS program - Orphan Drug designation, Fast Track designation, and Rare Pediatric Disease designation; and, for the Alzheimer’s Disease program - Regenerative Medicine Advanced Therapy (RMAT) designation and Fast Track designation.
2024年7月29日 - $Longeveron (LGVN.US)$週一美股盤前,該公司的股價上漲40.12%達到$4.82。該公司在費城召開的阿爾茨海默病國際會議上報告了其第2a期CLEAR MIND臨床試驗的積極結果。
Longeveron的產品Lomecel-B™️是一種細胞治療產品,旨在通過減少腦部炎症和減緩疾病發展來治療阿爾茨海默病。該試驗涉及48名輕度阿爾茨海默病患者,達到了主要的安全性目標,並在減緩認知和功能衰退方面顯示出了有希望的療效。Lomecel-B™️耐受性良好,無嚴重副作用,並且還與腦容積丟失的減少以及日常生活活動和患者生活質量的改善相關,這些改善由患者的護理人員觀察到。這些結果表明Lomecel-B™️可能是阿爾茨海默病有價值的未來治療方案。
“近年來,業界更加專注於推出新型阿爾茨海默病治療藥物,以治療每年患病的數百萬人”,Longeveron的首席醫療官Nataliya Agafonova萬.D.表示。“新的阿爾茨海默病治療藥物的出現表明我們越來越有能力治療曾被認爲無法治療的疾病,這一努力的下一步是開發一種既安全又有效的治療方案。第2a期CLEAR MIND試驗的結果令人鼓舞,證明了Lomecel-B™️在滿足這種需求方面的潛力,我期待繼續開發。”
關於Lomecel-B™️
 Lomecel-B™️是一種活細胞製品,由從年輕健康成年供體骨髓中分離的特殊細胞製成。這些特殊細胞稱爲藥物信號細胞(MSCs),是我們內源性生物修復機制的重要組成部分。MSCs已被證明在身體內執行許多複雜功能,包括新組織的形成。它們還被證明會響應受損或患病的部位,並分泌具有免疫調節和再生作用的生物活性因子。我們認爲Lomecel-B™️可能具有多種潛在的作用機制,可能導致抗炎、促血管再生反應,因此可能在一系列罕見和與衰老有關的疾病中具有廣泛的應用。
Lomecel-B™️是一種活細胞製品,由從年輕健康成年供體骨髓中分離的特殊細胞製成。這些特殊細胞稱爲藥物信號細胞(MSCs),是我們內源性生物修復機制的重要組成部分。MSCs已被證明在身體內執行許多複雜功能,包括新組織的形成。它們還被證明會響應受損或患病的部位,並分泌具有免疫調節和再生作用的生物活性因子。我們認爲Lomecel-B™️可能具有多種潛在的作用機制,可能導致抗炎、促血管再生反應,因此可能在一系列罕見和與衰老有關的疾病中具有廣泛的應用。
關於Longeveron Inc.:
Longeveron是一家臨床階段的生物技術公司,正在開發再生醫學產品以滿足未滿足的醫療需求。該公司的主要研究產品是Lomecel-B™️,這是一種源於年輕健康的成年供體骨髓中分離出的異體藥物信號細胞(MSC)治療產品。Lomecel-B™️具有多種潛在的作用機制,包括促進血管生成、促進再生、抗炎以及組織修復和癒合的作用,廣泛適用於一系列疾病領域。Longeveron目前正在追求三個潛在的治療方案:無左心室綜合症(HLHS)、阿爾茨海默病和與衰老有關的虛弱。Lomecel-B™️的開發計劃已獲得五個不同的FDA指定:對於HLHS項目 - 孤兒藥物指定、快速通道指定和罕見兒童疾病指定;對於阿爾茨海默病項目 - 再生醫學先進療法(RMAT)指定和快速通道指定。
 Lomecel-B™️是一種活細胞製品,由從年輕健康成年供體骨髓中分離的特殊細胞製成。這些特殊細胞稱爲藥物信號細胞(MSCs),是我們內源性生物修復機制的重要組成部分。MSCs已被證明在身體內執行許多複雜功能,包括新組織的形成。它們還被證明會響應受損或患病的部位,並分泌具有免疫調節和再生作用的生物活性因子。我們認爲Lomecel-B™️可能具有多種潛在的作用機制,可能導致抗炎、促血管再生反應,因此可能在一系列罕見和與衰老有關的疾病中具有廣泛的應用。
Lomecel-B™️是一種活細胞製品,由從年輕健康成年供體骨髓中分離的特殊細胞製成。這些特殊細胞稱爲藥物信號細胞(MSCs),是我們內源性生物修復機制的重要組成部分。MSCs已被證明在身體內執行許多複雜功能,包括新組織的形成。它們還被證明會響應受損或患病的部位,並分泌具有免疫調節和再生作用的生物活性因子。我們認爲Lomecel-B™️可能具有多種潛在的作用機制,可能導致抗炎、促血管再生反應,因此可能在一系列罕見和與衰老有關的疾病中具有廣泛的應用。
 Lomecel-B™️ is a living cell product made from specialized cells isolated from the bone marrow of young healthy adult donors. These specialized cells, known as medicinal signaling cells (MSCs), are essential to our endogenous biological repair mechanism. MSCs have been shown to perform a number of complex functions in the body, including the formation of new tissue. They also have been shown to respond to sites of injury or disease and secrete bioactive factors that are immunomodulatory and regenerative. We believe that Lomecel-B™️ may have multiple potential mechanisms of action that may lead to anti-inflammatory, pro-vascular regenerative responses, and therefore may have broad application for a range of rare and aging related diseases.
Lomecel-B™️ is a living cell product made from specialized cells isolated from the bone marrow of young healthy adult donors. These specialized cells, known as medicinal signaling cells (MSCs), are essential to our endogenous biological repair mechanism. MSCs have been shown to perform a number of complex functions in the body, including the formation of new tissue. They also have been shown to respond to sites of injury or disease and secrete bioactive factors that are immunomodulatory and regenerative. We believe that Lomecel-B™️ may have multiple potential mechanisms of action that may lead to anti-inflammatory, pro-vascular regenerative responses, and therefore may have broad application for a range of rare and aging related diseases.