Market Mover | Eli Lilly Shares Surge 12% as the Company Raises Full-Year Revenue Guidance by $3 Billion
Market Mover | Eli Lilly Shares Surge 12% as the Company Raises Full-Year Revenue Guidance by $3 Billion
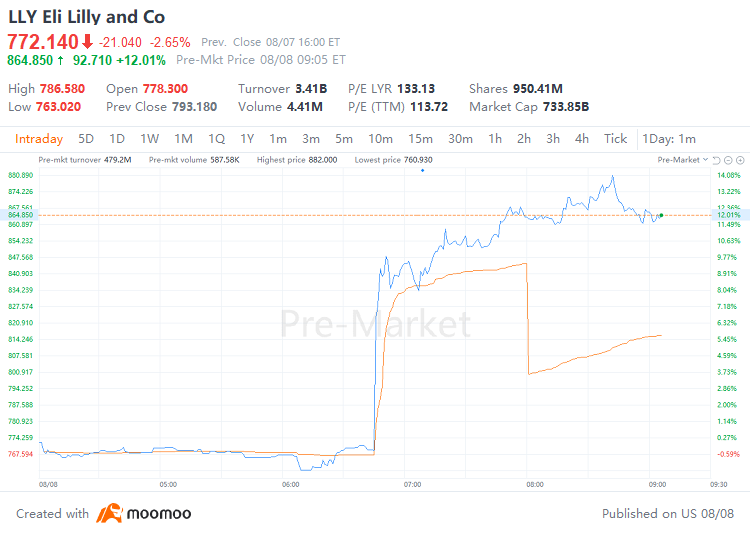
August 8, 2024 - $Eli Lilly and Co (LLY.US)$ shares surged 12.01% to $864.85 in pre-market trading on Thursday. The company today announced its financial results for the second quarter of 2024.
2024年8月8日 - $禮來 (LLY.US)$ 週四美股盤前,股價上漲12.01%至864.85美元。公司今天公佈了2024年第二季度的財務業績。
Q2 Highlights
Q2亮點
Revenue in Q2 2024 increased 36%, driven by Mounjaro, Zepbound and Verzenio. When excluding $579.0 million of revenue from the sale of rights for Baqsimi in Q2 2023, revenue in Q2 2024 increased 46%. Excluding the sale of rights for Baqsimi, non-incretin revenue increased 17% worldwide and 25% in the U.S.
Q2 2024 EPS increased 68% to $3.28 on a reported basis and increased 86% to $3.92 on a non-GAAP basis, both inclusive of $0.14 of acquired IPR&D charges.
2024 full-year revenue guidance raised by $3 billion; reported EPS guidance raised $2.05 to the range of $15.10 to $15.60, and non-GAAP EPS guidance raised $2.60 to the range of $16.10 to $16.60.
Pipeline progress included approval of Kisunla in the U.S. for Alzheimer's disease and Jaypirca in Japan for relapsed or refractory mantle cell lymphoma. Additional progress included submission of tirzepatide in the U.S. and EU for obstructive sleep apnea and obesity, and positive topline results from the Phase 3 trial evaluating tirzepatide for heart failure with preserved ejection fraction and obesity.
2024財年第二季度營業收入增長36%,由Mounjaro,Zepbound和Verzenio驅動。在不包括2023年第二季度Baqsimi版權銷售額的57900萬美元的情況下,2024財年第二季度營業收入增長46%。在不包括Baqsimi的版權銷售額的情況下,非增素收入在全球範圍內增長了17%,在美國增長了25%。
2024財年第二季度每股收益按報告基礎增長了68%,按非GAAP基礎增長了86%,均包括收購的知識產權研發費用的0.14美元。
2024年全年營業收入指引上調30億美元; 報告的EPS指引上調2.05美元,區間範圍爲15.10美元至15.60美元,非通用會計準則下的EPS指引上調2.60美元,區間範圍爲16.10美元至16.60美元。
管線進展包括 Kisunla 獲得美國治療老年癡呆症的批准,Jaypirca(用於反覆發作或難治性瀰漫性B細胞淋巴瘤)在日本獲得批准。其他進展包括提交美國和歐盟的tirzepatide,用於阻塞性睡眠呼吸暫停和肥胖症,並公佈了評估tirzepatide治療心力衰竭和肥胖症的第三期試驗的積極上線結果。
Lilly shared numerous updates recently on key regulatory, clinical, business development and other events, including:
利利最近就關鍵的監管、臨床、業務發展和其他事件分享了衆多更新,包括:
U.S. Food and Drug Administration (FDA) approval of Kisunla™️ (donanemab-azbt) for the treatment of Alzheimer's disease;
Approval of Jaypirca® in Japan for people with relapsed or refractory mantle cell lymphoma who are resistant or intolerant to other Bruton tyrosine kinase inhibitors.
Submission of tirzepatide in the U.S. and EU for the treatment of moderate-to-severe obstructive sleep apnea in adults with obesity.
Submission of mirikizumab in Japan for the treatment of moderately to severely active Crohn's disease.
Positive topline results from the SUMMIT Phase 3 clinical trial evaluating tirzepatide in adults with heart failure with preserved ejection fraction and obesity.
Positive topline results from the QWINT-2 and QWINT-4 Phase 3 clinical trials that showed once-a-week dosing of insulin efsitora alfa in adults with type 2 diabetes delivers A1C reduction and safety profile consistent with daily insulin.
The announcement of an agreement for Lilly to acquire Morphic Holding, Inc. to expand Lilly's immunology pipeline with oral integrin therapies for treatment of serious chronic diseases.
The commitment of an additional $5.3 billion manufacturing investment in the company's newest Indiana site to boost API production for tirzepatide and pipeline medicines.
The issuance of an open letter informing the public about potentially serious risks posed by the proliferation of counterfeit, fake, compounded, and other unsafe or untested versions of the company's FDA-approved tirzepatide medications and about the appropriate use of the company's authentic medicines.
美國食品和藥物管理局(FDA)批准 Kisunla™️(donanemab-azbt)用於治療阿爾茨海默病;
Jaypirca® 在日本獲得批准,用於治療轉歸或難治的幕上或復發幕上細胞淋巴瘤患者,這些患者對其他布通酪氨酸激酶抑制劑存在耐藥性或不耐受性
在美國和歐盟提交 tirzepatide 申請,用於治療患有肥胖症的成人中度至重度梗阻性睡眠呼吸暫停
在日本提交 mirikizumab 申請,用於治療中度至重度活動性克羅恩病
SUMMIt第3期臨床試驗結果爲陽性,該試驗評估成人心臟衰竭伴有保留射血分數和肥胖症患者的 tirzepatide
QWINt-2 和 QWINt-4 第 3 期臨床試驗是陽性的,這表明一週一次使用 Insulin efsitora alfa 降低成人 2 型糖尿病的 A1C 並提供與每日胰島素一致的安全性
宣佈達成一項協議,Lilly將收購 Morphic Holding, Inc.,以擴大Lilly的免疫學產品線,用於治療嚴重慢性疾病的口服整合素療法。
該公司在印第安納州最新的工廠中額外投資53億美元,以推動 Tirzepatide 和管道藥物的 API 生產。
發佈了一封公開信,告知公衆非法制造仿製、假冒、混合和其他不安全或未經檢驗的 Tirzepatide 藥物的潛在嚴重風險,以及關於公司真正藥物的正確使用。
2024 Financial Guidance
2024年全年財務指導意見
2024 full-year revenue guidance increased by $3.0 billion to the range of $45.4 billion to $46.6 billion, primarily driven by the strong performance of Mounjaro and Zepbound, as well as the company's non-incretin medicines. Additionally, the company has improved clarity into the timing and pace of the company's production expansions and planned Mounjaro launches outside the U.S. In Q2 2024, the company achieved a number of supply-related milestones and has increased confidence regarding production expectations for the rest of the year.
全年營業收入指引增加30億美元,區間範圍爲454億至466億美元,主要由Mounjaro、Zepbound的強勁表現以及公司的非腸高素藥物驅動。此外,該公司對公司的生產擴張和計劃在美國以外地區推出Mounjaro的時間和步伐有了更清晰的認識。在2024年第二季度,公司實現了許多供應相關的里程碑,並提高了對今年餘下時間生產預期的信心。
EPS guidance increased to the ranges of $15.10 to $15.60 on a reported basis and $16.10 to $16.60 on a non-GAAP basis.
EPS指引增加到報告式和非通用會計準則分別爲15.10美元至15.60美元,以及16.10美元至16.60美元的範圍。
Related Reading: Press Release
相關閱讀:新聞發佈
