Market Mover | Biohaven Shares Jump Over 10% After Announcing Positive Topline Results in Pivotal Study of Troriluzole in Spinocerebellar Ataxia
Market Mover | Biohaven Shares Jump Over 10% After Announcing Positive Topline Results in Pivotal Study of Troriluzole in Spinocerebellar Ataxia
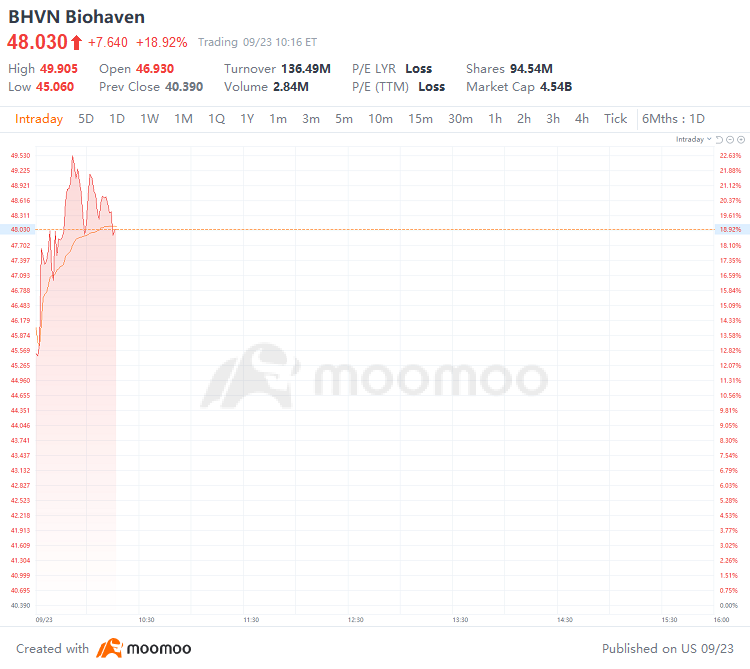
September 23, 2024 - $Biohaven (BHVN.US)$ rises 18.92% to $48.030 on Monday. The company achieves positive topline results in pivotal study of troriluzole in SCA.
2024年9月23日 - $Biohaven (BHVN.US)$ 週一,Biohaven(BHVN.US)上漲18.92%,至48.030美元。該公司在SCA的關鍵研究中取得了積極的上線結果。
What Happened?
發生了什麼?
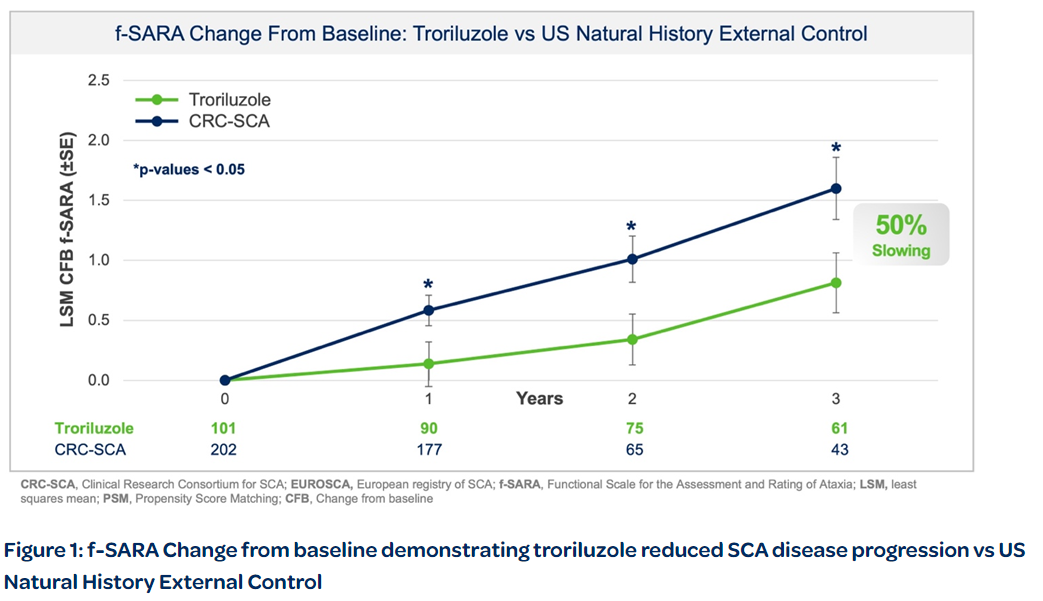
Biohaven Ltd. today announced positive topline results from pivotal Study BHV4157-206-RWE (NCT06529146) demonstrating the efficacy of troriluzole on the mean change from baseline in the f-SARA after 3 years of treatment. The study achieved the primary endpoint and showed statistically significant improvements on the f-SARA at years 1 and 2 (Figure 1). SCA is a rare, progressively debilitating neurodegenerative disease that affects approximately 15,000 people in the United States and 24,000 in Europe and the United Kingdom. There are no FDA approved treatments for SCA.
Biohaven Ltd.今天宣佈關鍵研究BHV4157-206-RWE(NCT06529146)取得了積極的上線結果,證明troriluzole對治療3年後f-SARA基線變化的有效性。該研究實現了主要終點,並在第1和第2年在f-SARA上顯示了統計顯著的改善(見圖1)。SCA是一種罕見逐漸惡化的神經退行性疾病,影響美國約15,000人,歐洲和英國約24,000人。目前尚無FDA批准的SCA治療方法。
Collectively, data across multiple analyses demonstrate a robust and clinically meaningful slowing of disease progression in SCA patients. These treatment benefits translate into a 50-70% slower rate of decline compared to untreated patients, representing 1.5-2.2 years delay in disease progression over the 3-year study period. Additionally, in a responder sensitivity analysis, disease progression when defined by a 2 point or greater worsening on the f-SARA at 3 years showed an odds ratio (OR) of 4.1 (95% CI: 2.1, 8.1) for the untreated external control arm versus troriluzole treated subjects (p < 0.0001; pooled analysis).
跨多個分析的數據合集顯示,對SCA患者疾病進展減緩具有強大且臨床意義重大的效果。這些治療效益轉化爲疾病進展速度比未治療患者慢50-70%,相當於在3年研究期間延遲疾病進展1.5-2.2年。此外,在一個反應敏感性分析中,根據f-SARA在3年內定義的2分或更大惡化來定義的疾病進展顯示,未經troriluzole治療的外部對照組與接受troriluzole治療的受試者(p
Statements by Relevant Practitioners
相關從業者的聲明
Dr. Susan Perlman, Director of Ataxia Clinic and Neurogenetics Clinical Trials at the David Geffen School of Medicine at UCLA stated, "SCA is a debilitating, relentlessly progressive disease that destroys quality of life, leaving patients unable to care for themselves, walk, or speak. Troriluzole is the very first treatment to show a delay in disease progression that can give patients additional years of independence, where they can walk without assistance, continue to work, play with their children, and participate in daily activities. This is an exciting and hopeful moment for the SCA community."
Dr. Susan Perlman,加州大學洛杉磯分校大衛蓋芬醫學院共濟失調診所和神經遺傳學臨床試驗主任表示:「SCA是一種毀滅性、持續進展的疾病,摧毀了生活質量,使患者無法自理、行走或言語。Troriluzole是首個能延緩疾病進展的治療方法,可以讓患者多過幾年的獨立自主,在這段時間裏他們可以自行行走、繼續工作、和孩子一起玩耍,並參與日常活動。對SCA社區而言,這是一個令人振奮和充滿希望的時刻。」
About the Company
關於公司
Biohaven Ltd. is a global clinical stage biopharmaceutical company, which engages in the discovery, development, and commercialization of therapies for people with neurological and neuropsychiatric diseases. The company was founded on May 2, 2022 and is headquartered in New Haven, CT.
Biohaven是一家全球臨床階段生物製藥公司,致力於發現、研發和商業化治療神經和神經精神疾病的新療法。該公司成立於2022年5月2日,總部位於康涅狄克州紐黑文。
For more information, please refer to Biohaven Achieves Positive Topline Results in Pivotal Study of Troriluzole in Spinocerebellar Ataxia (SCA)

 Collectively, data across multiple analyses demonstrate a robust and clinically meaningful slowing of disease progression in SCA patients. These treatment benefits translate into a 50-70% slower rate of decline compared to untreated patients, representing 1.5-2.2 years delay in disease progression over the 3-year study period. Additionally, in a responder sensitivity analysis, disease progression when defined by a 2 point or greater worsening on the f-SARA at 3 years showed an odds ratio (OR) of 4.1 (95% CI: 2.1, 8.1) for the untreated external control arm versus troriluzole treated subjects (p < 0.0001; pooled analysis).
Collectively, data across multiple analyses demonstrate a robust and clinically meaningful slowing of disease progression in SCA patients. These treatment benefits translate into a 50-70% slower rate of decline compared to untreated patients, representing 1.5-2.2 years delay in disease progression over the 3-year study period. Additionally, in a responder sensitivity analysis, disease progression when defined by a 2 point or greater worsening on the f-SARA at 3 years showed an odds ratio (OR) of 4.1 (95% CI: 2.1, 8.1) for the untreated external control arm versus troriluzole treated subjects (p < 0.0001; pooled analysis).