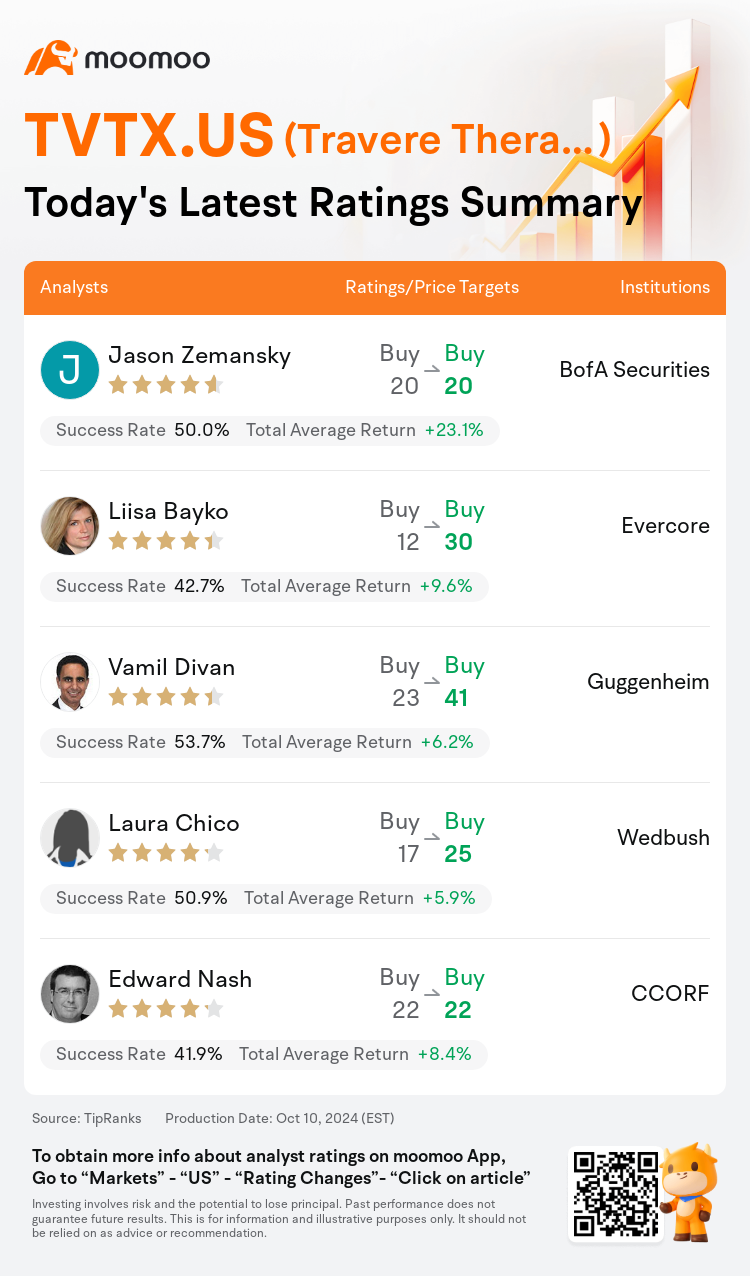
On Oct 10, major Wall Street analysts update their ratings for $Travere Therapeutic (TVTX.US)$, with price targets ranging from $20 to $41.
BofA Securities analyst Jason Zemansky maintains with a buy rating, and maintains the target price at $20.
Evercore analyst Liisa Bayko maintains with a buy rating, and adjusts the target price from $12 to $30.
 Guggenheim analyst Vamil Divan maintains with a buy rating, and adjusts the target price from $23 to $41.
Guggenheim analyst Vamil Divan maintains with a buy rating, and adjusts the target price from $23 to $41.
Wedbush analyst Laura Chico maintains with a buy rating, and adjusts the target price from $17 to $25.
CCORF analyst Edward Nash maintains with a buy rating, and maintains the target price at $22.
Furthermore, according to the comprehensive report, the opinions of $Travere Therapeutic (TVTX.US)$'s main analysts recently are as follows:
Following a recent scientific workshop, there is growing excitement within the medical community about the potential of using proteinuria as a primary endpoint in clinical trials. Should this be embraced by regulatory bodies, it would have decidedly favorable consequences for treatments targeting conditions such as focal segmental glomerulosclerosis (FSGS). In light of these developments, expectations are being cautiously adjusted to include a projected market introduction for such therapies, with conservative estimates anticipating a launch year and the likelihood of successful outcomes.
Following the recent PARASOL Working Group meeting in Bethesda, MD, which brought together representatives from various sectors to deliberate on endpoints for focal segmental glomerulosclerosis trials, two primary insights emerged. Firstly, there is an incentive to transition away from eGFR as a trial endpoint in FSGS. Secondly, proteinuria is being considered as a potential validated surrogate endpoint within these trials. It is anticipated that Travere will seek a meeting with the FDA and aim to submit an sNDA in the first half of 2025. The final results from PARASOL are expected to be shared at the ASN meeting and in a scholarly publication. Additionally, should approval be granted, it could enhance Travere's attractiveness as a potential M&A target.
The completion of the PARASOL workshop has increased confidence in the potential approval of Filspari for the treatment of focal segmental glomerulosclerosis (FSGS), resulting in an enhanced probability of success estimate for Filspari in treating FSGS to 75% from the previous 60%.
Here are the latest investment ratings and price targets for $Travere Therapeutic (TVTX.US)$ from 5 analysts:
Note:
TipRanks, an independent third party, provides analysis data from financial analysts and calculates the Average Returns and Success Rates of the analysts' recommendations. The information presented is not an investment recommendation and is intended for informational purposes only.
Success rate is the number of the analyst's successful ratings, divided by his/her total number of ratings over the past year. A successful rating is one based on if TipRanks' virtual portfolio earned a positive return from the stock. Total average return is the average rate of return that the TipRanks' virtual portfolio has earned over the past year. These portfolios are established based on the analyst's preliminary rating and are adjusted according to the changes in the rating.
TipRanks provides a ranking of each analyst up to 5 stars, which is representative of all recommendations from the analyst. An analyst's past performance is evaluated on a scale of 1 to 5 stars, with more stars indicating better performance. The star level is determined by his/her total success rate and average return.
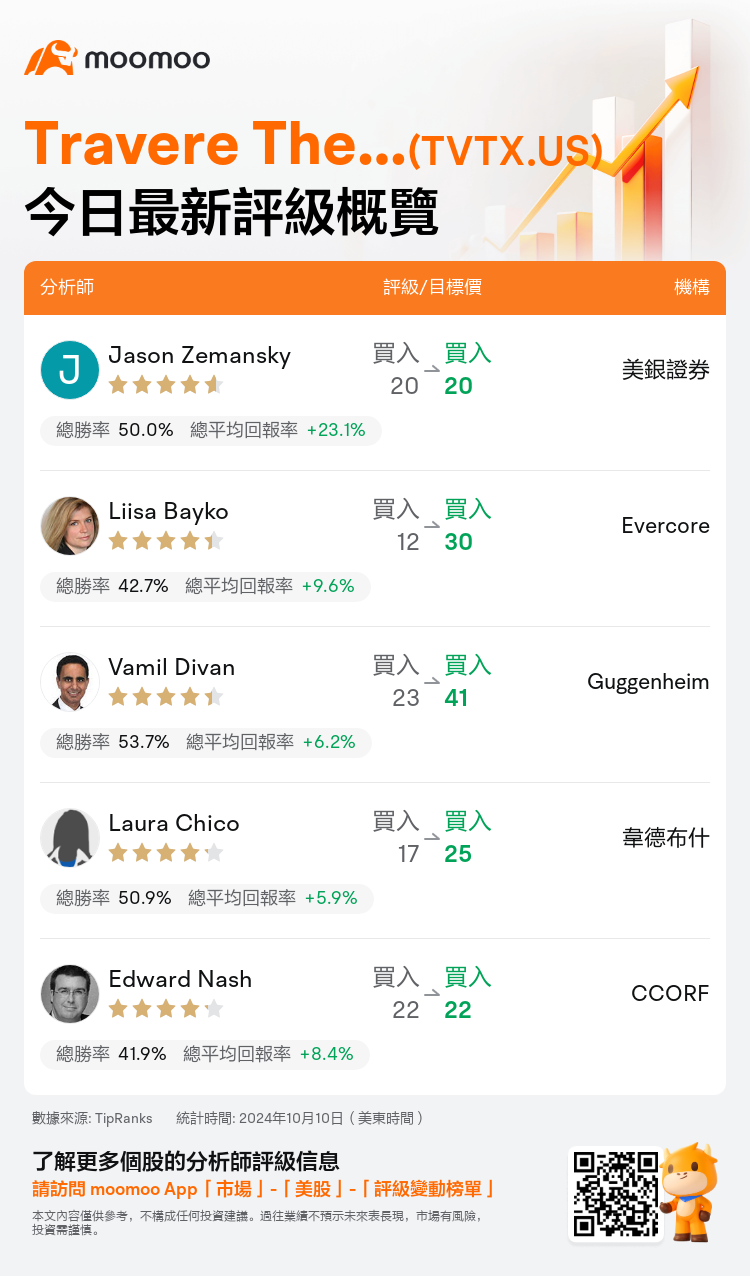
美東時間10月10日,多家華爾街大行更新了$Travere Therapeutic (TVTX.US)$的評級,目標價介於20美元至41美元。
美銀證券分析師Jason Zemansky維持買入評級,維持目標價20美元。
Evercore分析師Liisa Bayko維持買入評級,並將目標價從12美元上調至30美元。
 Guggenheim分析師Vamil Divan維持買入評級,並將目標價從23美元上調至41美元。
Guggenheim分析師Vamil Divan維持買入評級,並將目標價從23美元上調至41美元。
韋德布什分析師Laura Chico維持買入評級,並將目標價從17美元上調至25美元。
CCORF分析師Edward Nash維持買入評級,維持目標價22美元。
此外,綜合報道,$Travere Therapeutic (TVTX.US)$近期主要分析師觀點如下:
最近舉行的科學研討會後,醫療界對將蛋白尿作爲臨床試驗的主要終點的潛力日益興奮。如果監管機構採納這一做法,將會對治療瞄準類似節段性腎小球硬化(FSGS)的疾病產生明顯有利的後果。鑑於這些發展,人們謹慎調整了預期,包括對此類療法的市場推出進行了預測,保守估計推斷出一個上市年份和成功結果的可能性。
在馬里蘭州貝塞斯達(Bethesda)召開的PARASOL工作組會議後,彙集了各個領域的代表以研究類似節段性腎小球硬化試驗的終點,得出了兩個主要見解。首先,FSGS試驗將有轉向將eGFR作爲試驗終點的激勵。其次,在這些試驗中,蛋白尿被視爲潛在的驗證替代終點。預計Travere將尋求與FDA會面,並計劃在2025年上半年提交sNDA。PARASOL的最終結果預計將在ASN會議和學術刊物上公佈。此外,一旦獲得批准,這可能會增強Travere作爲潛在併購目標的吸引力。
PARASOL研討會的結束增加了對Filspari治療類似節段性腎小球硬化(FSGS)獲得批准的信心,導致Filspari在治療FSGS方面的成功概率評估從之前的60%提高到75%。
以下爲今日5位分析師對$Travere Therapeutic (TVTX.US)$的最新投資評級及目標價:
提示:
TipRanks為獨立第三方,提供金融分析師的分析數據,並計算分析師推薦的平均回報率和勝率。提供的信息並非投資建議,僅供参考。本文不對評級數據和報告的完整性與準確性做出認可、聲明或保證。
TipRanks提供每位分析師的星級,分析師星級代表分析師所有推薦的過往表現,通過分析師的總勝率和平均回報率综合計算得出,星星越多,則該分析師過往表現越優異,最高爲5颗星。
分析師總勝率為近一年分析師的評級成功次數占總評級次數的比率。評级的成功與否,取決於TipRanks的虚擬投資組合是否從該股票中產生正回報。
總平均回報率為基於分析師的初始評級創建虚擬投資組合,並根據評級變化對組合進行調整,在近一年中該投資組合所獲得的回報率。
 Guggenheim分析師Vamil Divan維持買入評級,並將目標價從23美元上調至41美元。
Guggenheim分析師Vamil Divan維持買入評級,並將目標價從23美元上調至41美元。 
 Guggenheim analyst Vamil Divan maintains with a buy rating, and adjusts the target price from $23 to $41.
Guggenheim analyst Vamil Divan maintains with a buy rating, and adjusts the target price from $23 to $41.