Intelligent Bio Solutions Announces Positive Results of PK Study, a Key Milestone on Path to FDA 510(k) Submission
Intelligent Bio Solutions Announces Positive Results of PK Study, a Key Milestone on Path to FDA 510(k) Submission
PK Study Successfully Demonstrates that Fingerprint Sweat Provides Reliable Sample for Drug Detection
Pk研究成功證明指紋汗液提供可靠的藥物檢測樣本
FDA 510(k) Clearance Would Enable the Introduction of INBS' Technology to the US in 2025
FDA 510(k)認證將使INBS的科技在2025年進入美國市場
NEW YORK, Nov. 13, 2024 (GLOBE NEWSWIRE) -- Intelligent Bio Solutions Inc. (Nasdaq: INBS) ("INBS" or the "Company"), a medical technology company delivering intelligent, rapid, non-invasive testing solutions, today announced strong initial results from its Pharmacokinetic (PK) study required for an FDA 510(k) submission. The data from the PK study shows that fingerprint sweat mimics the rate and extent of codeine in blood and saliva. The study successfully demonstrated that fingerprint sweat provides a reliable sample matrix for drug detection, showing quantitative PK data closely aligned to blood, based on statistical comparisons made at the 95% confidence level.
紐約,2024年11月13日(環球新聞社)- Intelligent Bio Solutions Inc.(納斯達克股票代碼:INBS)(「INBS」或「公司」),一家醫療技術公司,提供智能、快速、非侵入式的檢測解決方案,今天宣佈其爲FDA 510(k)提交所需的藥代動力學(PK)研究取得了強勁的初步結果。來自PK研究的數據顯示,指紋汗液模擬血液和唾液中可待因的速率和程度。該研究成功地證明了指紋汗液提供了一種可靠的藥物檢測樣本基質,顯示出量化的PK數據與血液緊密一致,基於在95%置信水平下進行的統計比較。
"The close correlation of PK parameters in fingerprint sweat and blood highlights its robustness as a sampling approach. While independent data has consistently supported this, our clinical study further reinforces these findings," said Harry Simeonidis, President and CEO of Intelligent Bio Solutions. "We are very pleased with the PK study results. This data highlights the potential for our technology to achieve widespread adoption in safety-critical industries and beyond, marking a significant achievement as we advance toward FDA clearance in the United States."
「指紋汗液和血液中的Pk參數密切相關,突出其作爲採樣方法的魯棒性。儘管獨立數據始終支持這一點,但我們的臨床研究進一步強化了這些發現,」智能生物解決方案公司的總裁兼首席執行官哈里·西梅奧尼斯表示。「我們對Pk研究結果感到非常滿意。這些數據凸顯了我們的技術在安全關鍵行業及其他領域廣泛應用的潛力,標誌着我們在向美國FDA申請認證的進程中取得了重要成就。」
FDA 510(k) clearance would enable INBS to introduce its innovative drug screening technology to the US market in 2025. The PK study results and other clinical data from the Company's clinical study plan will be submitted as part of the Company's 510(k) submission, expected in the fourth calendar quarter of this year. INBS is well-positioned to meet the increasing demand for drug testing in the United States, particularly in safety-critical industries like construction, mining, and transportation, as well as in law enforcement, drug rehabilitation, and forensic sectors.
FDA 510(k) 許可將使 INBS 能夠在 2025 年將其創新的藥物篩查技術引入美國市場。Pk 研究結果和其他臨床數據將作爲公司510(k)提交的一部分提交,預計將在今年第四個日曆季度進行。INBS 處於良好的位置,以滿足美國對藥物檢測日益增長的需求,特別是在施工、礦業和運輸等安全關鍵行業,以及在執法、藥物康復和法醫學領域。
Results: Average Codeine Values in Whole Blood, Oral Fluid and Fingerprint Sweat1
結果:全血、口液和指紋汗液中的平均可待因值1
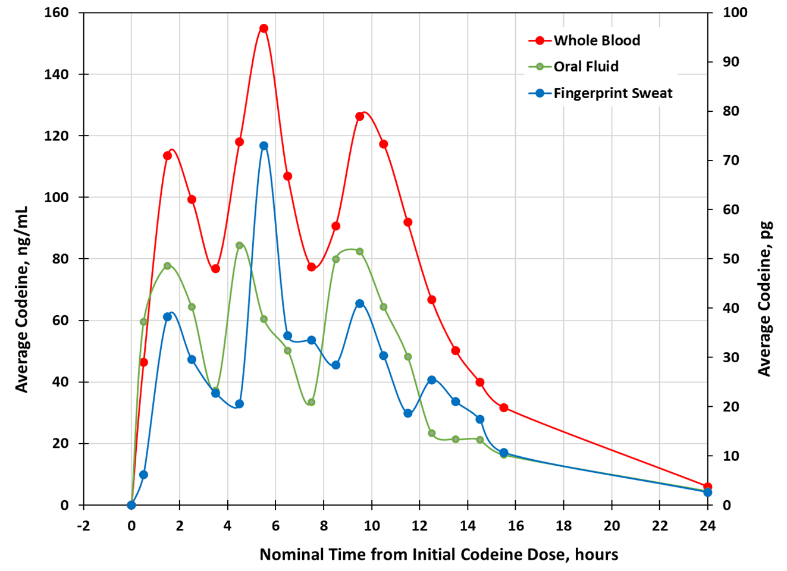
The study results show that fingerprint sweat is a strong indicator of codeine ingestion. They further validate the ability of INBS' method to provide rapid and reliable drug screening through a simple fingerprint sweat sample without invasive procedures.
研究結果顯示,指紋汗液是可待因攝入的重要指標。進一步驗證了 INBS 方法通過簡單的指紋汗液樣本在無侵入性程序下提供快速和可靠的藥物篩查的能力。
The PK study, conducted in partnership with Cliantha Research, required a minimum of 36 subjects. The Company successfully recruited 39 healthy adult subjects from diverse backgrounds, including varying genders, ages, and ethnicities. The study compared the levels of opiates detected in fingerprint sweat with those found in blood, oral fluid, and urine samples following the medically supervised administration of codeine. All fingerprint sweat specimens collected using INBS' Intelligent Fingerprinting Drug Screening System, were analyzed by a validated, traceable liquid chromatography/tandem mass spectrometry (LC-MS/MS) method, widely accepted as the gold standard for such studies.
與 Cliantha Research 合作進行的Pk研究,要求至少36名受試者。公司成功招募了39名來自不同背景的健康成人受試者,包括不同性別、年齡和種族。該研究比較了指紋汗液中檢測到的鴉片類藥物水平與在醫學監督下給予可待因後,在血液、口液和尿液樣本中檢測到的水平。使用INBS智能指紋藥物篩查系統收集的所有指紋汗液樣本,均通過一種經過驗證的可追溯液相色譜/串聯質譜(LC-MS/MS)方法進行分析,該方法被廣泛接受爲此類研究的黃金標準。
About Intelligent Bio Solutions
關於智能生物解決方案
Intelligent Bio Solutions Inc. (NASDAQ: INBS) is a medical technology company delivering innovative, rapid, non-invasive testing solutions. The Company believes that its Intelligent Fingerprinting Drug Screening System will revolutionize portable testing through fingerprint sweat analysis, which has the potential for broader applications in additional fields. Designed as a hygienic and cost-effective system, the test screens for the recent use of drugs commonly found in the workplace, including opiates, cocaine, methamphetamine, and cannabis. With sample collection in seconds and results in under ten minutes, this technology would be a valuable tool for employers in safety-critical industries. The Company's current customer segments outside the US include construction, manufacturing and engineering, transport and logistics firms, drug treatment organizations, and coroners.
納斯達克:Intelligent Bio Solutions Inc. (INBS)是一家醫療技術公司,提供創新、快速、非侵入性的測試解決方案。該公司認爲其Intelligent Fingerprinting毒品篩查系統將通過指紋汗液分析革新便攜式測試,這具有在其他領域中有更廣泛應用潛力。設計爲衛生和經濟實惠的系統,該測試旨在篩查最近在工作場所常見的藥物使用,包括阿片類藥物、可卡因、冰毒和大麻。採樣只需幾秒鐘,結果在不到十分鐘內出來,這項技術將成爲安全關鍵行業僱主的寶貴工具。該公司目前在美國以外的客戶群包括建築、製造和工程、運輸和物流公司、藥物治療機構和法醫。
For more information, visit:
請訪問:
Forward-Looking Statements
前瞻性聲明
Some of the statements in this release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995, which involve risks and uncertainties. Forward-looking statements in this press release include, without limitation, Intelligent Bio Solutions Inc.'s ability to successfully develop and commercialize its drug and diagnostic tests, realize commercial benefit from its partnerships and collaborations, and secure regulatory approvals, among others. Although Intelligent Bio Solutions Inc. believes that the expectations reflected in such forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Intelligent Bio Solutions Inc. has attempted to identify forward-looking statements by terminology, including "believes," "estimates," "anticipates," "expects," "plans," "projects," "intends," "potential," "may," "could," "might," "will," "should," "approximately" or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, included in Intelligent Bio Solutions' public filings filed with the Securities and Exchange Commission. Any forward-looking statements contained in this release speak only as of its date. Intelligent Bio Solutions undertakes no obligation to update any forward-looking statements contained in this release to reflect events or circumstances occurring after its date or to reflect the occurrence of unanticipated events.
智能生物解決方案股份有限公司。
Company Contact:
公司聯繫方式:
Intelligent Bio Solutions Inc.
info@ibs.inc
LinkedIn | Twitter
智能生物解決方案股份有限公司。
info@ibs.inc
領英 | 推特
Investor & Media Contact:
投資者及媒體聯繫方式:
Valter Pinto, Managing Director
KCSA Strategic Communications
PH: (212) 896-1254
INBS@kcsa.com
Valter Pinto, 董事總經理
KCSA戰略傳播
電話:(212)896-1254
INBS@kcsa.com
1 Urine results are not included in this chart. Due to the nature of urine sample collection, which depends on an individual's need to urinate, samples cannot be collected at fixed intervals. Therefore, urine specimens were collected on an ad-lib basis as frequently as possible.
1 尿液結果未包含在此圖表中。由於尿液樣本收集的性質取決於個體的排尿需求,因此樣本無法在固定的間隔內收集。因此,尿液標本是在儘可能頻繁的情況下按需收集的。
A photo accompanying this announcement is available at
本公告附有一張照片。

 "The close correlation of PK parameters in fingerprint sweat and blood highlights its robustness as a sampling approach. While independent data has consistently supported this, our clinical study further reinforces these findings," said Harry Simeonidis, President and CEO of Intelligent Bio Solutions. "We are very pleased with the PK study results. This data highlights the potential for our technology to achieve widespread adoption in safety-critical industries and beyond, marking a significant achievement as we advance toward FDA clearance in the United States."
"The close correlation of PK parameters in fingerprint sweat and blood highlights its robustness as a sampling approach. While independent data has consistently supported this, our clinical study further reinforces these findings," said Harry Simeonidis, President and CEO of Intelligent Bio Solutions. "We are very pleased with the PK study results. This data highlights the potential for our technology to achieve widespread adoption in safety-critical industries and beyond, marking a significant achievement as we advance toward FDA clearance in the United States."