MannKind Shares Are Falling Today: What's Going On?
MannKind Shares Are Falling Today: What's Going On?
MannKind Corporation (NASDAQ:MNKD) are trading lower Monday. The company released its six-month results from its Phase 3 INHALE-1 pediatric diabetes trial. Here's what you need to know.
週一,曼肯德公司(納斯達克股票代碼:MNKD)股價走低。該公司公佈了其三期 INHALE-1 兒科糖尿病試驗的六個月結果。以下是你需要知道的。
What To Know: The trial investigated the use of Afrezza (insulin human) Inhalation Powder in children and adolescents aged 4 to 17 years. Its primary endpoint was a non-inferior change in HbA1c levels after 26 weeks of treatment.
須知:該試驗調查了Afrezza(人胰島素)吸入粉末在4至17歲兒童和青少年中的使用情況。其主要終點是治療26周後HbA1c水平的變化不遜色。
The results showed that, while Afrezza met the non-inferiority criteria based on a modified intent-to-treat (mITT) analysis, a full intent-to-treat (ITT) analysis did not meet the predetermined non-inferiority margin, largely due to the variability introduced by one non-compliant patient. Despite this, the mITT analysis supported Afrezza's non-inferiority to multiple daily injections (MDI) of rapid-acting insulin.
結果顯示,儘管根據修改後的意向治療(MiTT)分析,Afrezza符合非劣勢標準,但全面的意向治療(ITT)分析並未達到預先確定的非劣勢範圍,這主要是由於一名不合規患者引入了變異性。儘管如此,MiTT的分析支持Afrezza並不遜色於每日多次注射速效胰島素(MDI)。
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
該研究還顯示,Afrezza和MDI組之間的肺功能沒有顯著差異,兩個治療組在26周內保持穩定的FEV1水平。兩種治療方案之間沒有發現任何重大的安全問題,包括低血糖。
Despite the encouraging efficacy and safety outcomes, the stock's decline reflects investor cautiousness, as the company now plans to meet with the U.S. Food and Drug Administration (FDA) in the first half of 2025 regarding a supplemental new drug application (sNDA) for Afrezza's use in pediatric populations. This meeting is crucial for the potential next steps in expanding the treatment's approval, which may contribute to further volatility in the stock price depending on the regulatory response.
儘管療效和安全結果令人鼓舞,但該股的下跌反映了投資者的謹慎態度,因爲該公司現在計劃在2025年上半年與美國食品藥品監督管理局(FDA)會面,討論Afrezza在兒科人群中使用的補充新藥申請(snDa)。這次會議對於在擴大該療法批准範圍方面可能採取的下一步措施至關重要,這可能會導致股價進一步波動,具體取決於監管部門的應對措施。
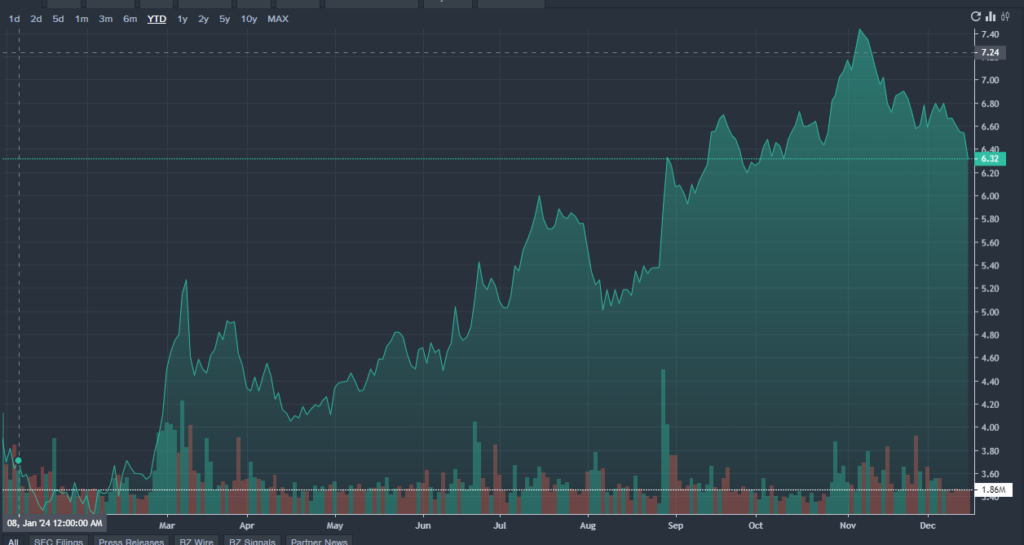
MNKD Price Action: MannKind shares were down 4.28% at $6.27 at the time of writing, according to Benzinga Pro.
MNKD價格走勢:根據Benzinga Pro的數據,在撰寫本文時,MannKind股價下跌4.28%,至6.27美元。
- Nvidia And 5 Other Stocks Are Analyst's Top Semiconductor Picks For 2025, Sees 2 AI Trends
- 英偉達和其他5只股票是分析師2025年的首選半導體,預計有2種人工智能趨勢
Image Via Shutterstock.
圖片來自 Shutterstock。

 The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.
The study also showed no significant differences in lung function between the Afrezza and MDI groups, with both treatment groups maintaining stable FEV1 levels over the 26-week period. No major safety concerns, including hypoglycemia, were noted between the two treatment regimens.