Eli Lilly's Zepbound Receives FDA Approval For Obesity-Linked Sleep Apnea Drug
Eli Lilly's Zepbound Receives FDA Approval For Obesity-Linked Sleep Apnea Drug
On Friday, the U.S. Food and Drug Administration approved Eli Lilly and Co.'s (NYSE:LLY) weight loss drug Zepbound for treating sleep apnea
週五,美國食品藥品監督管理局批准了禮來(NYSE:LLY)公司的減肥藥Zepbound用於治療睡眠 apnea。
What Happened: The FDA's decision makes Zepbound the first approved drug treatment for patients with obesity and moderate-to-severe obstructive sleep apnea, or OSA.
發生了什麼:FDA的決定使Zepbound成爲第一個獲得批准的用於肥胖及中度至重度阻塞性睡眠 apnea(OSA)患者的藥物治療。
"Too often, OSA is brushed off as 'just snoring,'" said Julie Flygare, CEO of Project Sleep, in Eli Lilly's press release. The condition affects an estimated 80 million Americans, with 20 million experiencing moderate-to-severe forms.
「阻塞性睡眠 apnea常常被忽視爲『僅僅是打鼾』,」項目睡眠的首席執行官Julie Flygare在禮來的新聞稿中表示。該控制項影響大約8000萬美國人,其中2000萬人經歷中度至重度的症狀。
Weight Loss Drug Wars Heat Up: Amgen Challenges Eli Lilly, Novo's Dominance
減肥藥物戰爭白熱化:安進對艾 Eli Lilly 和諾和諾德的主導地位發起挑戰
In a separate release, the FDA underscored that Zepbound should be used in conjunction with a reduced-calorie diet and increased physical activity for optimal results.
在另一份聲明中,FDA強調Zepbound應與低熱量飲食和增加的身體活動結合使用以獲得最佳效果。
The approval could significantly impact insurance coverage for Zepbound, potentially including Medicare, which currently restricts coverage for obesity drugs unless approved for additional health benefits, noted CNBC.
此批准可能會對Zepbound的保險覆蓋產生重大影響,潛在地包括醫療保險,而目前醫療保險對肥胖藥物的覆蓋有限,除非獲得額外健康利益的批准,CNBC指出。
This development also gives Eli Lilly an edge over competitor Novo Nordisk (NYSE:NVO), whose Wegovy isn't approved for sleep apnea treatment.
這一進展還使得禮來在競爭對手諾和諾德(NYSE:NVO)面前佔據優勢,後者的Wegovy尚未獲得睡眠 apnea治療的批准。
Why It Matters: Zepbound's effectiveness was demonstrated in clinical trials. Results showed that nearly half of patients treated with the highest dose experienced disease resolution, the report noted.
爲什麼這很重要:Zepbound的有效性在臨牀試驗中得到了證明。結果顯示,接受最高劑量治療的患者中有近一半經歷了疾病的緩解,報告指出。
This was defined by a significant reduction in their apnea-hypopnea index (AHI), a key metric in diagnosing OSA.
這通過他們的呼吸暫停-低通氣指數(AHI)的顯著降低來定義,這是診斷阻塞性睡眠呼吸暫停的重要指標。
The approval also comes as Eli Lilly continues to expand its manufacturing capabilities, including a $3 billion investment in Kenosha County, Wisconsin in December, to meet the growing demand for its diabetes and obesity medicines.
此批准也恰逢禮來繼續擴大其製造業能力,包括在12月份對威斯康星州肯諾沙縣的30億元投資,以滿足對其糖尿病和肥胖藥物的日益增長的需求。
In October, Eli Lilly reported third-quarter revenue of $11.44 billion, a 20% increase year-over-year, but missed expectations of $12.10 billion. Zepbound generated $1.26 billion.
在10月份,禮來報告第三季度營業收入爲114.4億元,同比增長20%,但未達到121億元的預期。Zepbound產生了12.6億元。
Price Action: As per Benzinga Pro, LLY shares increased by 1.35% to $767.76 on Friday. So far this year, LLY has risen by 29.65%.
價格動態:根據Benzinga Pro,LLY股價週五上漲了1.35%,至767.76美元。截至目前,今年LLY股價上漲了29.65%。
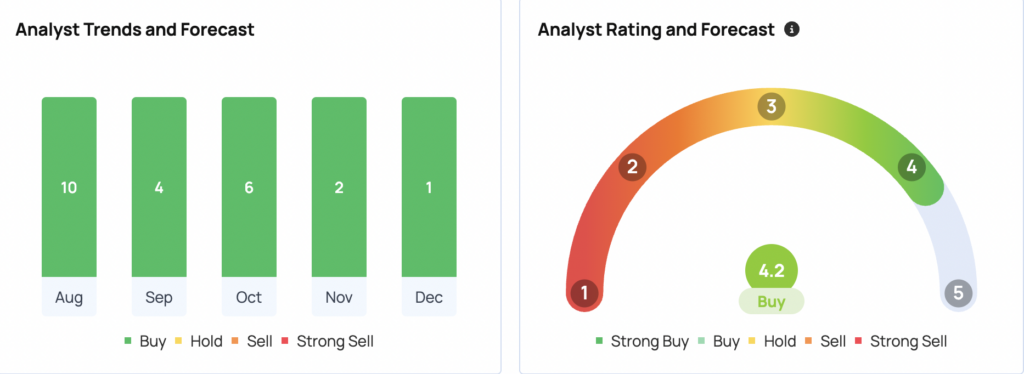
The latest ratings from B of A Securities, Wolfe Research, and Deutsche Bank set an average price target of $1,004, indicating a potential upside of 30.77%.
美銀證券、沃爾弗研究和德意志銀行的最新評級設定了1004美元的平均價格目標,表示潛在上漲幅度爲30.77%。
- Eli Lilly's Alzheimer's Drug Raises Hopes but Faces Regulatory Hurdles in Europe
- 伊莉莉的阿爾茨海默病藥物引發希望,但在歐洲面臨監管障礙
Disclaimer: This content was partially produced with the help of Benzinga Neuro and was reviewed and published by Benzinga editors.
免責聲明:此內容部分由Benzinga Neuro生成,並由Benzinga編輯審核和發佈。
Photo courtesy: Shutterstock
照片提供:Shutterstock

