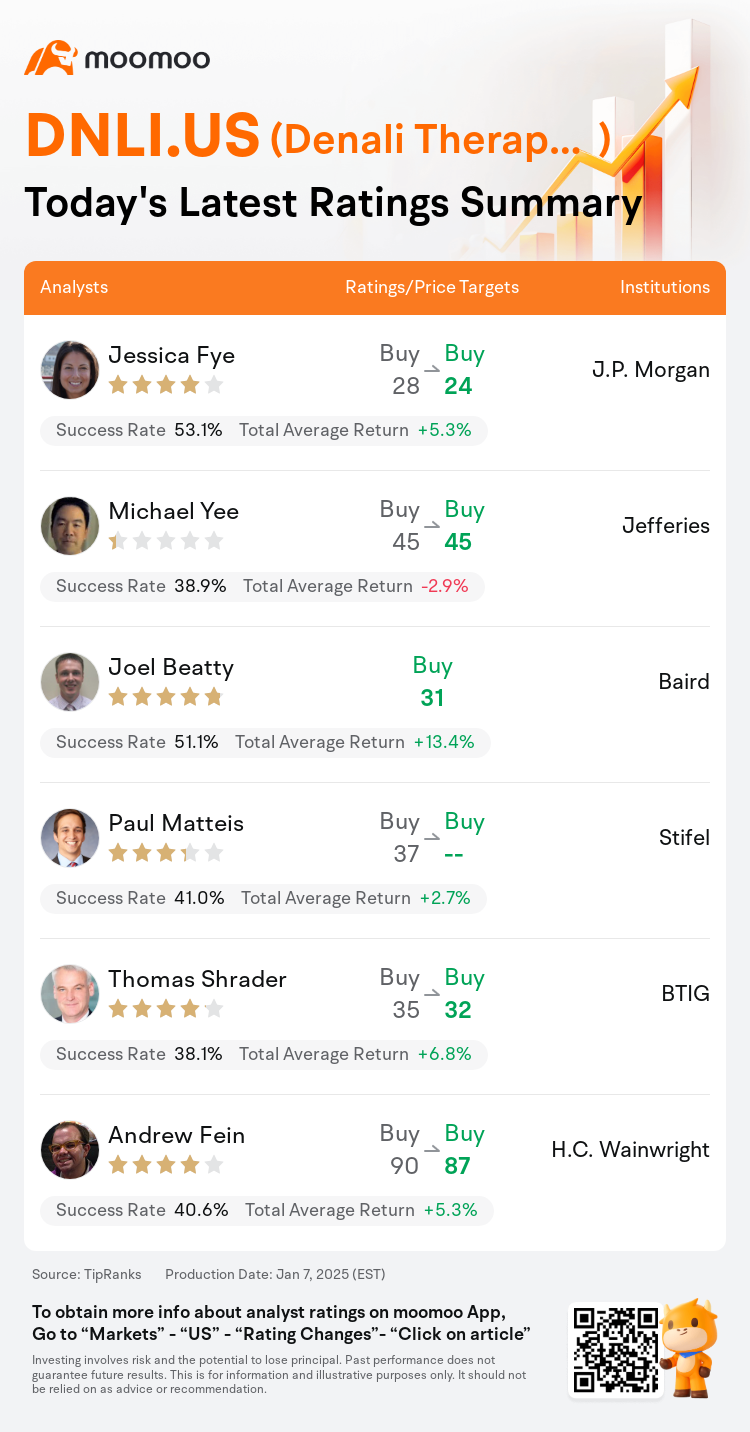
On Jan 07, major Wall Street analysts update their ratings for $Denali Therapeutics (DNLI.US)$, with price targets ranging from $24 to $87.
J.P. Morgan analyst Jessica Fye maintains with a buy rating, and adjusts the target price from $28 to $24.
Jefferies analyst Michael Yee maintains with a buy rating, and maintains the target price at $45.
 Baird analyst Joel Beatty initiates coverage with a buy rating, and sets the target price at $31.
Baird analyst Joel Beatty initiates coverage with a buy rating, and sets the target price at $31.
Stifel analyst Paul Matteis maintains with a buy rating.
BTIG analyst Thomas Shrader maintains with a buy rating, and adjusts the target price from $35 to $32.
Furthermore, according to the comprehensive report, the opinions of $Denali Therapeutics (DNLI.US)$'s main analysts recently are as follows:
The recent results from the Phase 2/3 HEALEY trial, testing an agonist of eIF2B for amyotrophic lateral sclerosis, highlighted the failure of DNL343. Despite the strong pre-clinical evidence suggesting reduced aggregation of major ALS pathology drivers such as TDP-43, the outcome was disappointing. Additionally, this was one of the last legacy programs at Denali, with a stated company focus shifting towards TV-oriented development pipeline in the future.
Denali Therapeutics is positioned to transform into a commercial entity by late 2025 or early 2026, largely thanks to the anticipated expedited FDA approval of its forefront treatment, tividenofusp alfa, designed for Hunter syndrome. This enzyme replacement therapy's ability to penetrate the brain suggests it could swiftly capture a notable portion of the market currently served by Elaprase, which generates $700M in annual sales. Additionally, Denali's early-stage 'Peak 2' projects aimed at addressing Alzheimer's and Parkinson's disease represent promising ventures for future growth.
Here are the latest investment ratings and price targets for $Denali Therapeutics (DNLI.US)$ from 6 analysts:
Note:
TipRanks, an independent third party, provides analysis data from financial analysts and calculates the Average Returns and Success Rates of the analysts' recommendations. The information presented is not an investment recommendation and is intended for informational purposes only.
Success rate is the number of the analyst's successful ratings, divided by his/her total number of ratings over the past year. A successful rating is one based on if TipRanks' virtual portfolio earned a positive return from the stock. Total average return is the average rate of return that the TipRanks' virtual portfolio has earned over the past year. These portfolios are established based on the analyst's preliminary rating and are adjusted according to the changes in the rating.
TipRanks provides a ranking of each analyst up to 5 stars, which is representative of all recommendations from the analyst. An analyst's past performance is evaluated on a scale of 1 to 5 stars, with more stars indicating better performance. The star level is determined by his/her total success rate and average return.
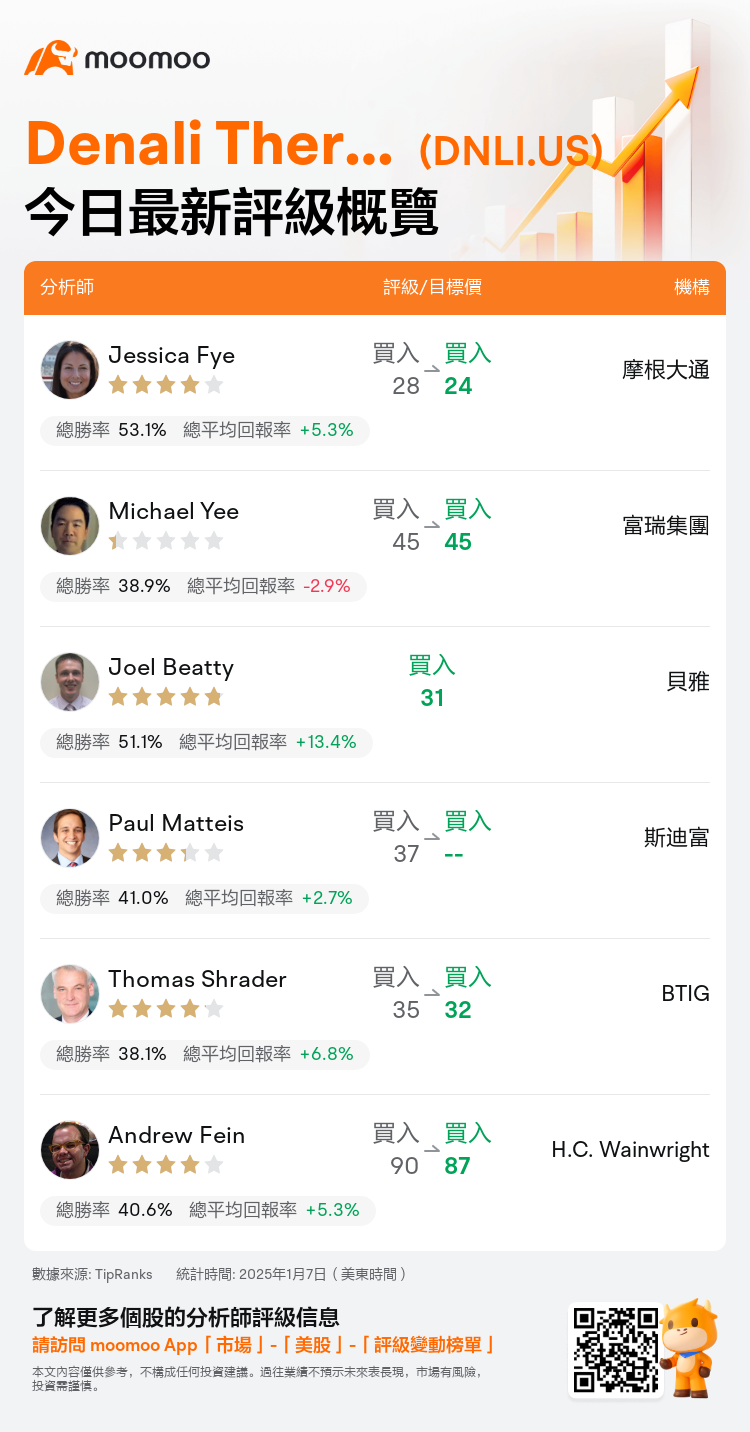
美東時間1月7日,多家華爾街大行更新了$Denali Therapeutics (DNLI.US)$的評級,目標價介於24美元至87美元。
摩根大通分析師Jessica Fye維持買入評級,並將目標價從28美元下調至24美元。
富瑞集團分析師Michael Yee維持買入評級,維持目標價45美元。
 貝雅分析師Joel Beatty首次給予買入評級,目標價31美元。
貝雅分析師Joel Beatty首次給予買入評級,目標價31美元。
斯迪富分析師Paul Matteis維持買入評級。
BTIG分析師Thomas Shrader維持買入評級,並將目標價從35美元下調至32美元。
此外,綜合報道,$Denali Therapeutics (DNLI.US)$近期主要分析師觀點如下:
最近的HEALEY二期/三期試驗結果測試了一種針對肌萎縮側索硬化症的eIF20億激動劑,突出了DNL343的失敗。儘管有強有力的前臨牀證據表明減少了重大ALS病理驅動因數如TDP-43的聚集,但結果令人失望。此外,這是Denali的最後幾個遺留項目之一,該公司的重點已轉向未來以電視爲導向的發展管道。
Denali Therapeutics預計將在2025年底或2026年初轉型爲一個商業實體,這主要得益於其前沿治療方案tividenofusp ALFA的FDA加速批准,該方案旨在治療亨特綜合症。這種酶替代療法能夠穿透大腦,表明它可以迅速捕獲目前由Elaprase提供的市場的顯著份額,Elaprase的年銷售額爲70000萬美元。此外,Denali的早期階段「Peak 2」項目旨在解決阿爾茨海默病和帕金森病,爲未來增長代表了有前景的投資。
以下爲今日6位分析師對$Denali Therapeutics (DNLI.US)$的最新投資評級及目標價:
提示:
TipRanks為獨立第三方,提供金融分析師的分析數據,並計算分析師推薦的平均回報率和勝率。提供的信息並非投資建議,僅供参考。本文不對評級數據和報告的完整性與準確性做出認可、聲明或保證。
TipRanks提供每位分析師的星級,分析師星級代表分析師所有推薦的過往表現,通過分析師的總勝率和平均回報率综合計算得出,星星越多,則該分析師過往表現越優異,最高爲5颗星。
分析師總勝率為近一年分析師的評級成功次數占總評級次數的比率。評级的成功與否,取決於TipRanks的虚擬投資組合是否從該股票中產生正回報。
總平均回報率為基於分析師的初始評級創建虚擬投資組合,並根據評級變化對組合進行調整,在近一年中該投資組合所獲得的回報率。
 貝雅分析師Joel Beatty首次給予買入評級,目標價31美元。
貝雅分析師Joel Beatty首次給予買入評級,目標價31美元。 
 Baird analyst Joel Beatty initiates coverage with a buy rating, and sets the target price at $31.
Baird analyst Joel Beatty initiates coverage with a buy rating, and sets the target price at $31.