On July 25, Mirxes Holding Company Limited (hereafter: Mirxes Holding Company Limited) submitted listing application documents to the Hong Kong Stock Exchange. CICC and CCB International are its co-sponsors.

Photo Source: Prospectus
 Since its establishment, Mirui has carried out many rounds of financing.The post-investment valuation of Series D financing is about 600 million US dollars.
Since its establishment, Mirui has carried out many rounds of financing.The post-investment valuation of Series D financing is about 600 million US dollars.
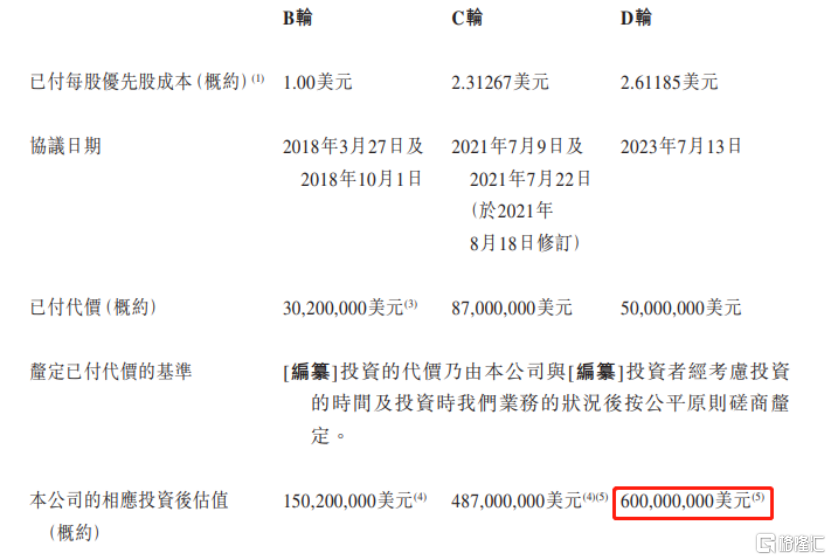
Photo Source: Prospectus
As of the date of signing of the prospectus, shareholders holding 5% or more of the company's shares mainly include Central Road, Dr. Zhu, Gene Limited, SLW, Accurate Gene Limited, MSEA Ltd, and Perpetuity Eagle International Ltd., holding 22.03%, 14.11%, 8.12%, 7.77%, 6.60%, and 5.19% of the shares, respectively.
Additionally, Rock Springs Capital, Koyong Capital, Zhongyuan, Mitsui & Co., Ltd., and CCB International are all shareholders of the company.
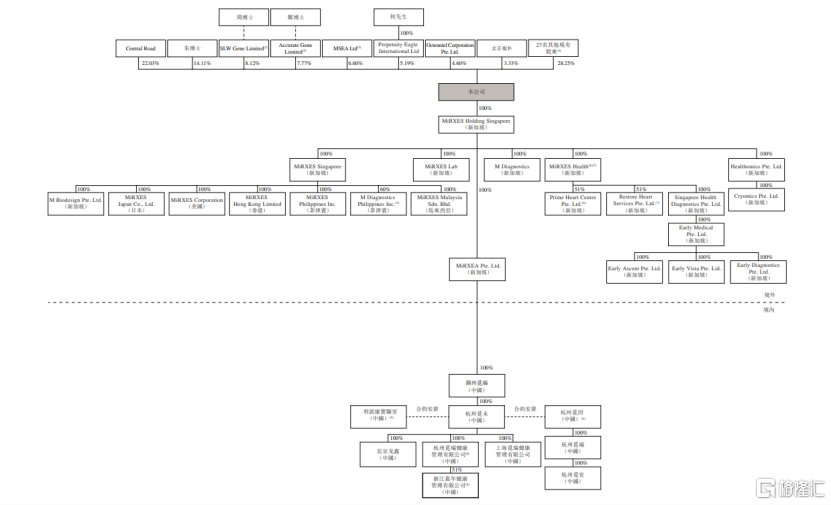
(Shareholding structure diagram, image source: prospectus)
1. Commercialize existing products
Founded in 2014, Mirui is a Singapore-based ribonucleic acid (“RNA”) technology company focused on popularizing early disease detection diagnostic solutions around the world.
The company's co-founder collaborated with other research institutions in early 2000 to establish Singapore's first PCR laboratory for RNA diagnosis. According to data from Frost & Sullivan, they then set up a world-leading miRNA candidate discovery laboratory in Singapore in 2012. The daily PCR reaction throughput was 0.2 million times, making it one of the highest miRNA candidate discovery laboratories in the world at the time.
At present, the company has developed two business segments, namely (a) Early Detection and Precise Multidisciplinary Division. Under this division, the company provides products and services for early detection of various diseases, multi-omics candidate detection and clinical genomic testing, and (b) infectious disease division.
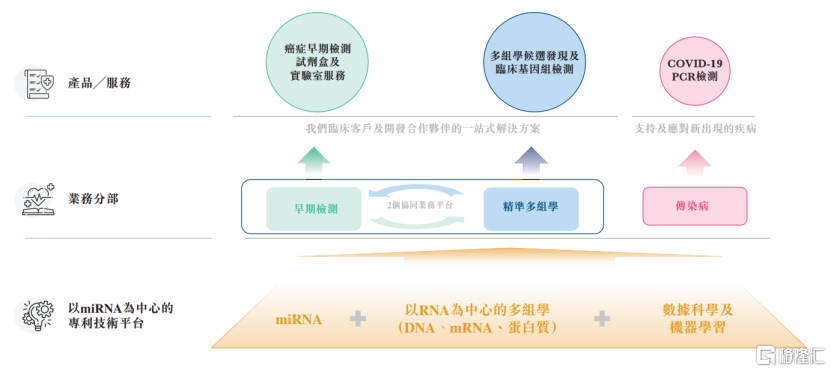
Photo Source: Prospectus
Among them, Minerui's core products come from the early detection business segment. The company mainly focuses on developing, producing and commercializing miRNA early detection kit products that can enter the mass market.
According to the prospectus, the company's core product, GastroClearTM, is the first and only model in the world to be approvedStomach cancer screeningThe molecular diagnostic IVD product is a blood-based miRNA test group composed of 12 miRNA biomarkers for gastric cancer screening and early detection. It was successfully commercialized after obtaining a Class C IVD certificate from HSA in September 2019.
In terms of market competition, in terms of revenue in 2022, GastroClearTM has the largest market share in the gastric cancer screening market in Southeast Asia.
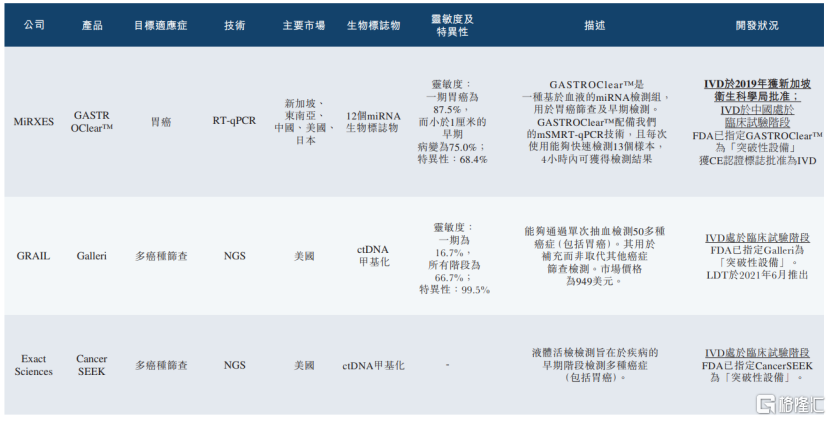
(Market competition situation of the company's core products, image source: prospectus)
Prospective clinical trial results show thatThe overall sensitivity of this product is about 87.0%However, the significant sensitivities for stage I gastric cancer and for early lesions smaller than 1 cm were 87.5% and 75.0%, respectively.
In May 2023, GastroClearTM received the FDA's breakthrough medical device title, making the company the first company to receive the title of FDA breakthrough medical device in the field of blood-based miRNA diagnostic testing and molecular diagnostic testing for gastric cancer.
Notably, the company has also developed a comprehensive early detection package for blood miRNA-based test kit products for cancer and cardiovascular diseases with high morbidity and mortality rates.Currently, only LungClearTM has commercialized LungClearTM as an LDT service in Southeast Asia, China, and Japan; the rest of the products are still in the early development stage.
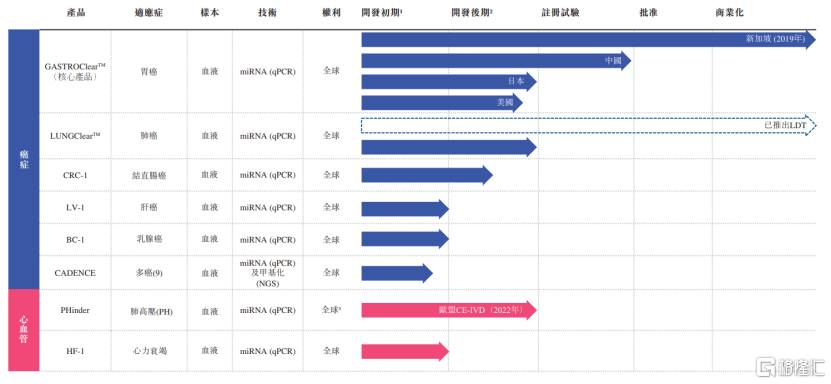
(Overview of the company's products and candidate products under the early testing platform, image source: prospectus)
In addition, the company's commercialized products include a FortitudeTM kit, which is one of the world's first approved COVID-19 RT-qPCR test kits. It was temporarily authorized by HSA for clinical use in March 2020, obtained CE certification in June 2020, and has been commercialized since then.
2. Gross margin continues to decline
In terms of business performance, in 2021, 2022, and January-April 2023, Minerui's earnings were approximately US$60.6498 million, US$17.759 million, and US$5.7751 million, respectively, during the corresponding year/periodThe amount of losses was US$3,0811 million, US$56.2027 million, and US$21.5871 million, respectively.
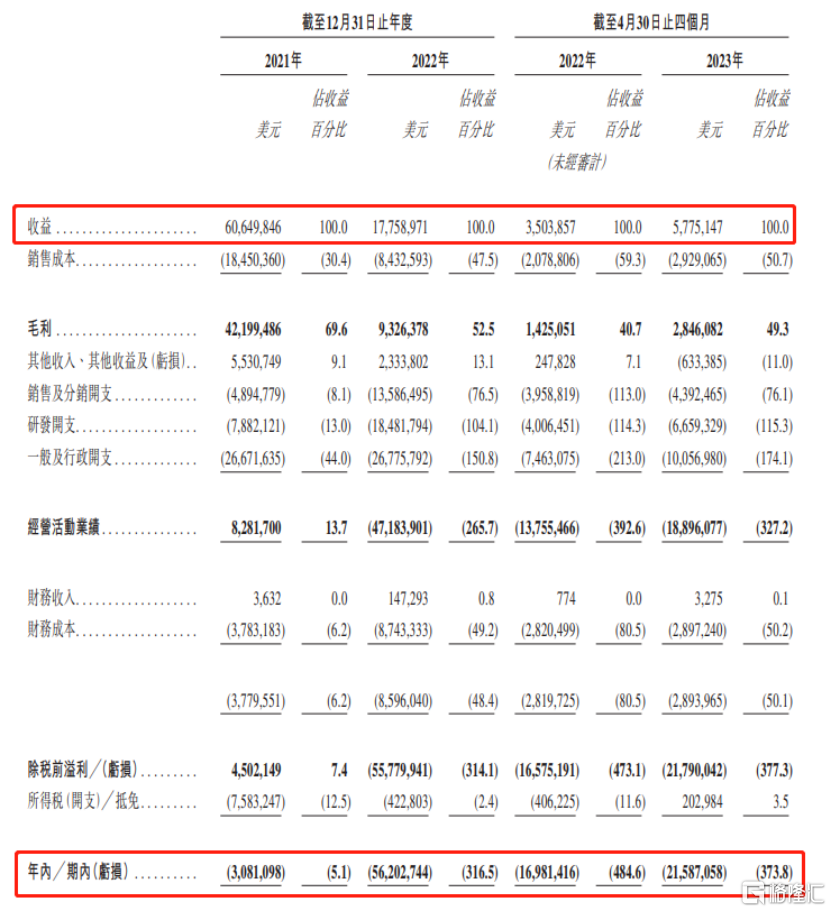
(Summary of comprehensive profit and loss and other comprehensive income statements, image source: prospectus)
It can be seen that the company's earnings in 2022 fell 70% year-on-year compared to 2021.The amount of losses increased dramatically.
The reason for this is that in 2021, nearly 90% of the company's revenue came from the Infectious Disease Business Division (FortitudeTM kit), but as the COVID-19 epidemic was mitigated in 2022 and the government began lifting COVID-related restrictions and measures, the demand for the company's FortitudeTM kits declined rapidly.Revenue from this business fell 86.2% year on year in 2022The share fell to 42.2%, and fell further to 20.8% from January to April 2023.
Looking at it this way,The company's future earnings will depend on further sales and commercialization of GastroClear and other candidate products in the Early Detection and Precise Multiomics business segment. If the product fails to meet the expected sales volume, pricing level, or profit margin in the future, it will have a significant adverse impact on the company's business, financial status and operating performance.
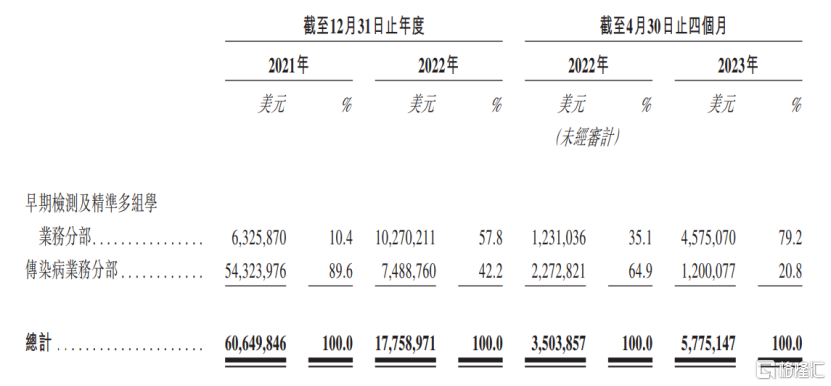
(Breakdown of earnings by segment, image source: prospectus)
Since most of the company's candidate products are still in the design or clinical development stage, the company invests most of its time and financial resources in the development and commercialization of existing candidate products. In the reporting period, R&D expenses generated by the company accounted for 13.0%, 104.1% and 115.3% of total revenue, respectively; sales and distribution expenses accounted for 8.1%, 76.5% and 76.1% of total revenue for the same period, respectively.
For each reporting period of 2021, 2022, and January-April 2023, the company'sGross margins were 69.6%, 52.5%, and 49.3% respectively, showing a continuous downward trend. Among them, the company drastically wrote off inventory related to COVID-19 test kits in 2022, resulting in a relatively low gross margin for selling diagnostic kits and other products.
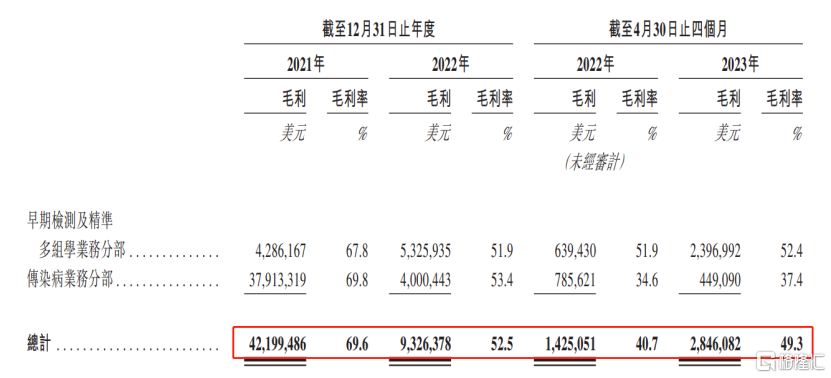
(Breakdown of gross profit and gross margin by division, image source: prospectus)
3. Conclusion
Overall, although Minerui already has commercialized products, the company's overall revenue fluctuates greatly. Market demand for one of these products has declined sharply, and the revenue scale of core products is still small. In the future, we still need to actively promote increased penetration of core products, while also expanding R&D capabilities and platforms and promoting pipeline products to enhance the company's profitability.

 自成立以来,觅瑞进行了多轮融资,D轮融资投后估值约6亿美元。
自成立以来,觅瑞进行了多轮融资,D轮融资投后估值约6亿美元。