Valued at over 4.5 billion yuan.
It has been learned from Gelonghui that recently, Yao Jie Ankang (Nanjing) Technology Co., Ltd. (hereinafter referred to as “Yao Jie Ankang”) submitted a prospectus to the Hong Kong Stock Exchange, intending to be listed on the main board of Hong Kong. Citic Securities and Huatai International are joint sponsors.
Yao Jie Ankang is a clinical-demand-oriented, registered clinical-stage biopharmaceutical company dedicated to discovering and developing small molecule innovative therapies for tumors, inflammation, and cardiovascular metabolic disorders.
In terms of equity structure, as of the date of signing the prospectus, Dr. Wu Yongqian held about 34.29% of the voting rights of Yao Jie Ankang through himself, Nanjing Yilu and Nanjing Jiminrui. Dr. Wu Yongqian joined the company in 2016, is now 61 years old, and has 27 years of experience in biopharmaceutical companies.
In this IPO, the funds raised by Yao Jie Ankang will be used to provide funds for the research and development of the core product Tinengotinib; provide funds for the research and development of other pipeline products; and provide general working capital and general company uses.
Two-year losses were nearly 0.6 billion yuan.
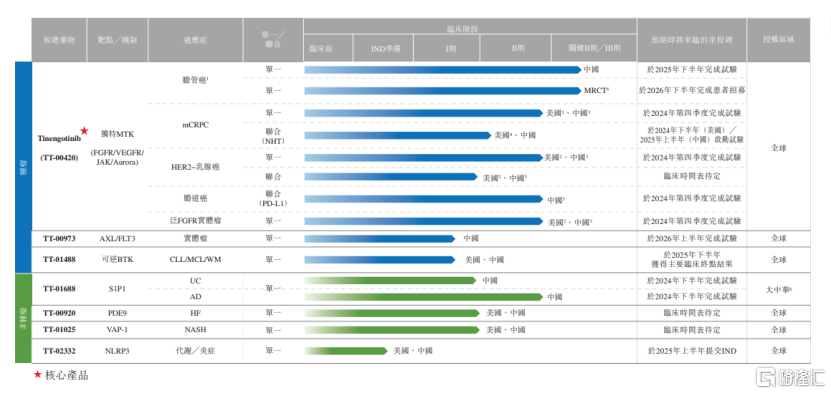
According to the prospectus, Yao Jie Ankang has six clinical-stage candidate products and one preclinical candidate product pipeline.
Among the seven product pipelines, Tinengotinib (TT-00420) is the core product of Yao Jie Ankang and is currently the closest pipeline to commercialization. It has entered phase 2 clinical trials in China and is used to treat bile duct cancer, breast cancer, cholangiocarcinoma, and other cancers.
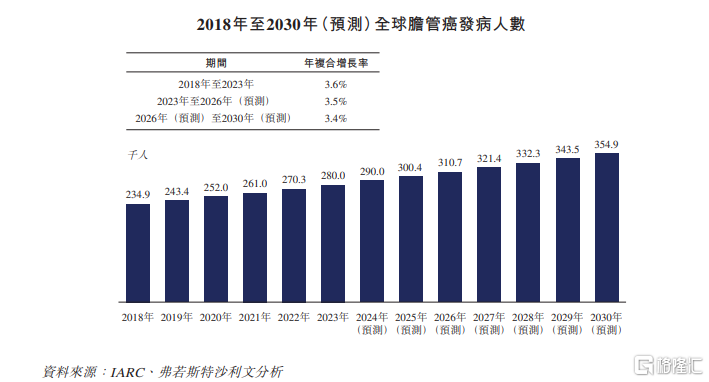
Biliary system cancer is a common type of liver and biliary cancer worldwide, usually including cholangiocarcinoma and gallbladder cancer. The bile duct is a branched channel that connects the liver and gallbladder to the small intestine. Bile duct cancer is a disease in which malignant cells form in the bile duct. Common symptoms include jaundice, fatigue, and abdominal pain.
In terms of industry scale, according to Frost Sullivan's statistics, the global biliary tract cancer drug market is expected to grow to $3.1 billion by 2026, and further to $5.4 billion by 2030, with a compound annual growth rate of 15.1% from 2026 to 2030.
In 2022, the market size of biliary tract cancer drugs in China reached 2 billion yuan, with a compound annual growth rate of 8.3% from 2018 to 2022. It is expected to further increase to 5.5 billion yuan and 10.6 billion yuan in 2026 and 2030, respectively.
In terms of financial data, due to the fact that the core drug has not yet achieved commercialization, Yao Jie Ankang is still deeply in losses.
According to the prospectus, in 2022 and 2023, the company's income was about 0.124 million yuan and 1.181 million yuan, respectively; during the same period, the company's net loss was about 0.252 billion yuan and 0.343 billion yuan, respectively.

Yao Jie Ankang stated that the losses are mainly caused by research and development costs and management expenses. The company expects to continue to lose money in the future, and as the number and scope of R&D projects expand, and as the company seeks regulatory approval and establishes a commercialization team, losses are expected to increase. Future net losses of the company depend on the number, scope, and cost of R&D projects, commercialization costs, and revenue-generating capabilities.
If candidate drugs fail, fail to obtain regulatory approval or market recognition, it may never be profitable. Even if profitable, it may not be sustainable. In addition, if the company fails to achieve or maintain profitability, it will reduce the company's value and weaken fundraising, R&D, expansion or maintenance capabilities.
In 2022 and 2023, Yao Jie Ankang’s R&D expenses were approximately RMB 0.263 billion and RMB 0.344 billion. Among them, the R&D expenses for the core product Tinengotinib were approximately RMB 0.167 billion and RMB 0.236 billion, respectively.
Yao Jie Ankang stated that clinical drug development is a long, costly, and uncertain process. The results of early and preliminary clinical trials may not predict the results of later or final trials, and the candidate drugs in later clinical trials may not demonstrate ideal safety and efficacy characteristics. Even if early trials produce satisfactory results, later clinical trials may still encounter major setbacks, and future clinical trial results may not be ideal.
On the one hand, the core drugs have not yet achieved commercialization and cannot contribute to the sources of income, and on the other hand, as an innovative drug company, it needs to continuously invest funds in research and development. The company's cash flow consumption is relatively large.
As of the end of 2023, Yao Jie Ankang's cash and cash equivalents on account have rapidly decreased from approximately 0.984 billion yuan at the end of 2022 to approximately 0.497 billion yuan at the end of 2023.
Valued at over 4.5 billion yuan.
Along the way, external financing has been an important driver for Yao Jie Ankang's development.
According to the prospectus, the company has completed nine rounds of financing, with a total financing amount of more than 1.7 billion yuan. Before the IPO, the company's investors included CIC Capital, Nation InvestCom, State Development Fund, Jinpu Investment, and others. The most recent round was D+ round financing in February 2023, with a funding amount of approximately 0.26 billion yuan and a post-investment valuation of approximately 4.59 billion yuan.

Innovative drug companies generally face challenges of large R&D investment, long cycles, and high risks, thus, losses become a common problem for these companies.
Another challenge is fierce competition in the development and commercialization of new drugs.
Ya Tai Jian Kang indicated that the company is facing competition from major pharmaceutical, specialty pharmaceutical, and biopharmaceutical companies worldwide. Numerous other companies are marketing, selling or developing drugs that this company is also researching. Some competitors have better resources and expertise. Potential competitors also include academic institutions, government agencies and other research institutions. It is expected that competition will become even fiercer with the launch of new drugs and technological advances.
If competitors' drugs are more effective, with fewer side effects, more convenient or cheaper, the company's business opportunities may be reduced. They may obtain regulatory approval and establish market positions before our company. Competitors may also render the company's candidate drugs obsolete or non-competitive, while the company has not yet recovered its development costs.
In addition, industry mergers and acquisitions may concentrate resources on a few competitors. Small and start-up companies may also become important competitors, competing with the company for talent, clinical trial resources and technology.
It is worth noting that Ya Tai Jian Kang has indicated that the company partly relies on third-party and its own licensed intellectual property, including patents, knowledge and proprietary technology. The company has entered into license agreements with third parties, and may enter into additional such agreements in the future, to acquire various third-party intellectual property. Termination of any such licenses may cause the company to lose rights and have an adverse effect on the company's ability to commercialize candidate drugs.
In addition, changes in international trade policies may also have adverse effects on the company's business and operating results. The company has established global strategic partnerships with a range of global pharmaceutical companies, including Roche, LG Chem, Teijin and EA Pharmaceuticals.
The company has conducted or plans to conduct parallel clinical trials for some candidate drugs in China, the United States, the European Union and other regions. Therefore, the company's business is influenced by changing international economic, regulatory, social and political conditions, as well as local environments in overseas countries and regions.
Epilogue
As a biopharmaceutical company in the clinical stage of registration, Ya Tai Jian Kang is still suffering losses. Fierce competition in the development and commercialization of new drugs puts pressure on Ya Tai Jian Kang from major global pharmaceutical companies. Changes in international trade policies may also affect the company's business, all of which are issues that the company needs to address.
