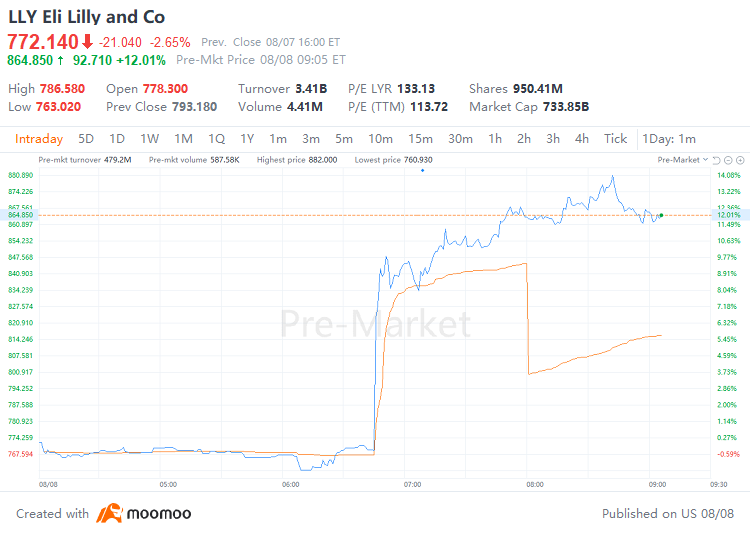
August 8, 2024 - $Eli Lilly and Co (LLY.US)$ shares surged 12.01% to $864.85 in pre-market trading on Thursday. The company today announced its financial results for the second quarter of 2024.
Q2 Highlights
Revenue in Q2 2024 increased 36%, driven by Mounjaro, Zepbound and Verzenio. When excluding $579.0 million of revenue from the sale of rights for Baqsimi in Q2 2023, revenue in Q2 2024 increased 46%. Excluding the sale of rights for Baqsimi, non-incretin revenue increased 17% worldwide and 25% in the U.S.
Q2 2024 EPS increased 68% to $3.28 on a reported basis and increased 86% to $3.92 on a non-GAAP basis, both inclusive of $0.14 of acquired IPR&D charges.
 2024 full-year revenue guidance raised by $3 billion; reported EPS guidance raised $2.05 to the range of $15.10 to $15.60, and non-GAAP EPS guidance raised $2.60 to the range of $16.10 to $16.60.
2024 full-year revenue guidance raised by $3 billion; reported EPS guidance raised $2.05 to the range of $15.10 to $15.60, and non-GAAP EPS guidance raised $2.60 to the range of $16.10 to $16.60.Pipeline progress included approval of Kisunla in the U.S. for Alzheimer's disease and Jaypirca in Japan for relapsed or refractory mantle cell lymphoma. Additional progress included submission of tirzepatide in the U.S. and EU for obstructive sleep apnea and obesity, and positive topline results from the Phase 3 trial evaluating tirzepatide for heart failure with preserved ejection fraction and obesity.
Lilly shared numerous updates recently on key regulatory, clinical, business development and other events, including:
U.S. Food and Drug Administration (FDA) approval of Kisunla™️ (donanemab-azbt) for the treatment of Alzheimer's disease;
Approval of Jaypirca® in Japan for people with relapsed or refractory mantle cell lymphoma who are resistant or intolerant to other Bruton tyrosine kinase inhibitors.
Submission of tirzepatide in the U.S. and EU for the treatment of moderate-to-severe obstructive sleep apnea in adults with obesity.
Submission of mirikizumab in Japan for the treatment of moderately to severely active Crohn's disease.
Positive topline results from the SUMMIT Phase 3 clinical trial evaluating tirzepatide in adults with heart failure with preserved ejection fraction and obesity.
Positive topline results from the QWINT-2 and QWINT-4 Phase 3 clinical trials that showed once-a-week dosing of insulin efsitora alfa in adults with type 2 diabetes delivers A1C reduction and safety profile consistent with daily insulin.
The announcement of an agreement for Lilly to acquire Morphic Holding, Inc. to expand Lilly's immunology pipeline with oral integrin therapies for treatment of serious chronic diseases.
The commitment of an additional $5.3 billion manufacturing investment in the company's newest Indiana site to boost API production for tirzepatide and pipeline medicines.
The issuance of an open letter informing the public about potentially serious risks posed by the proliferation of counterfeit, fake, compounded, and other unsafe or untested versions of the company's FDA-approved tirzepatide medications and about the appropriate use of the company's authentic medicines.
2024 Financial Guidance
2024 full-year revenue guidance increased by $3.0 billion to the range of $45.4 billion to $46.6 billion, primarily driven by the strong performance of Mounjaro and Zepbound, as well as the company's non-incretin medicines. Additionally, the company has improved clarity into the timing and pace of the company's production expansions and planned Mounjaro launches outside the U.S. In Q2 2024, the company achieved a number of supply-related milestones and has increased confidence regarding production expectations for the rest of the year.
EPS guidance increased to the ranges of $15.10 to $15.60 on a reported basis and $16.10 to $16.60 on a non-GAAP basis.
Related Reading: Press Release
