Net profit increased by 175% year-on-year, exceeding expectations, but the market's extreme pessimism has not yet been eliminated.
The heat of ADC is also reflected in the financial report of Joinn Laboratories.
Net income increased by 175.5% year-on-year, and unfilled orders increased by 105% year-on-year, surpassing the previous market's pessimistic expectations.
Industry beta brings growth, but the company is also working hard to acquire more customers.
 On the evening of August 20, 2024, Joinn Laboratories released the financial report for the first half of 2024, announcing that the company achieved revenue of 1.665 billion RMB, a year-on-year increase of 67.6%.
On the evening of August 20, 2024, Joinn Laboratories released the financial report for the first half of 2024, announcing that the company achieved revenue of 1.665 billion RMB, a year-on-year increase of 67.6%.
The high revenue growth comes from the industry beta, mainly due to the growth in demand for ADC and non-specific conjugate drug outsourcing, and more projects entering the later stage of development.
During the same period, the gross profit increased by 133.4% year-on-year, to 0.535 billion RMB, with a gross margin of 32.1%, an increase of 9.0 percentage points compared to the same period in 2023.
The increase in gross profit comes from revenue growth, capacity climbing, and cost control and procurement strategy optimization.
The most noteworthy thing is that the company's net income surpassed market expectations and reached 0.488 billion RMB, an increase of 175.5% YoY; net margin was 29.3%, an increase of 11.5 percentage points compared to the same period in 2023. Adjusted net income increased by 146.6% to 0.534 billion RMB, with an adjusted net margin of 32.0%, an increase of 10.2 percentage points compared to the same period in 2023.
The net income growth rate far exceeds the growth rate of the group's revenue and business, mainly benefiting from the rapid growth of revenue, along with further improvement in operating efficiency and more effective cost control. As a result, the group's net margin is 29.3%, an increase of 11.5 percentage points compared to the same period last year.
There has also been a breakthrough in new projects and customer data.
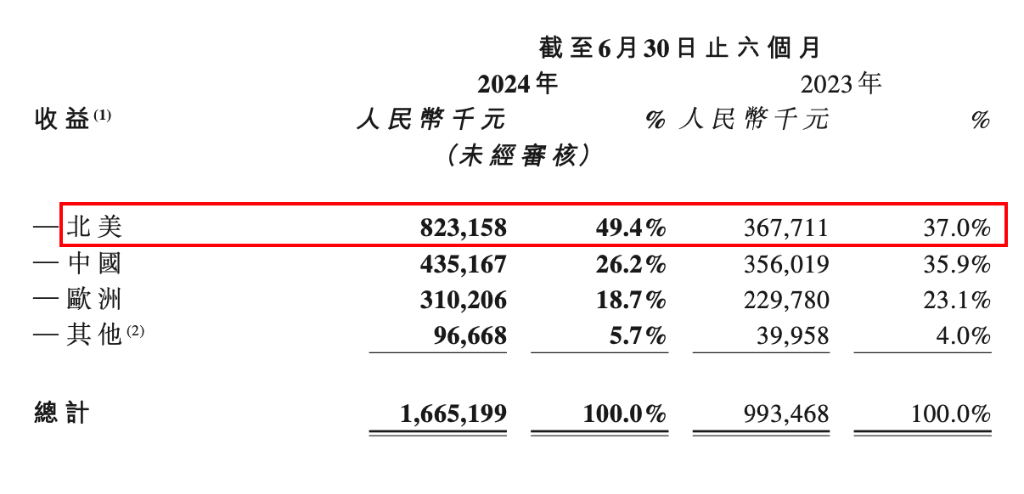
As of the end of the reporting period, the number of global customers for ChemPartner has increased to 419, with 71 new customers added in the first half of 2024. Among the top 20 global pharmaceutical companies, 13 have collaborated with the company on projects. In terms of revenue contribution, the proportion of North American customers has increased from 37% last year to 49.4% this year. The company mentioned during the conference call that the proportion of North American projects for the full year is expected to be above 40%.
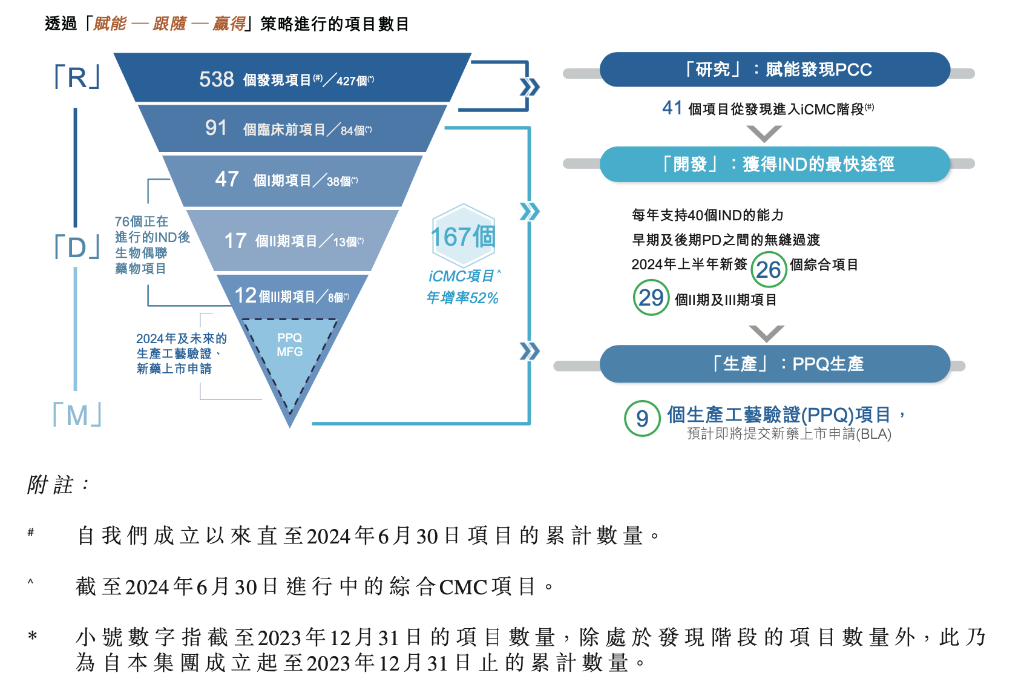
The 'empower, follow, and win the molecule' strategy has successfully driven sustained rapid growth in projects, with the total number of projects increasing to 167, including 26 new comprehensive projects signed in the first half of 2024.
The number of late-stage projects (Phase II and III clinical trials) that contribute more to performance has increased to 29, with 9 of them having the potential to submit BLAs in 2024 and beyond, particularly in relation to PPQ (Process Performance Qualification), potentially leading to more approved ADC products.
It is worth mentioning that during the conference call, the company stated that out of these 9 PPQ projects, some of them include projects won in the later stages of development.
Uncompleted orders increased by 105% YoY to 0.842 billion US dollars, and based on the order completion cycle, it is expected to ensure at least a double-digit growth in the next two years.
However, the company did not provide a specific breakdown of the sources of new signed orders, nor did it provide specific guidance, but management believes that the growth trend will continue, and the company's market share in the CMC phase should be the highest in the world.
The high prosperity of ADC is still ongoing.
In the first half of 2024, there are over 700 ADC/XDC projects in phases 2 and later globally, with 4 submitting BLAs (marketing applications), over 130 in phase 3, and 540 in phase 2. Especially in phase 2, more target products are rapidly advancing, driving the surge in ADC outsourcing demand.

In addition, the combination therapy of IO+ADC is also accelerating the demand for ADC products.

A concrete manifestation of the high prosperity in the industry is the continuous recruitment of employees by the company. As of June 30, 2024, Pharmaron LiAlliance had 1,496 full-time employees, a 72.2% increase compared to June 30, 2023.
The company expects to increase its staff to over 2,000 by the end of 2024. Previously, the guidance in the annual report aimed for 1,600-1,800 employees by the end of 2024. This upward adjustment in staff numbers expectation indicates that the global demand for ADC is still growing beyond expectations.
Pharmaron LiAlliance also revealed that the growth of ADC is comprehensive from R-D-M.
One detail also reflects the industry's upcoming trend. Li Jincai, CEO of Pharmaron LiAlliance, stated that although there have been no new ADC products approved globally for about a year and a half, in reality, a large number of pipelines have reached later stages, with a significant increase in demand for the manufacturing end. The upcoming demand will be even stronger, possibly leading to a capacity gap.
In this regard, it can also be seen from the perspective of production capacity planning that in the second half of the year, Pharmaron Alliance will accelerate capital expenditure to meet customer demands.
Based on the guidance given by management at the beginning of the year, the company's annual capital expenditure is estimated to be about $1.5 billion, with $0.5 billion spent in the first half of the year. Therefore, there will be around $1 billion of capital expenditure in the second half of the year.
Specifically, Pharmaron Alliance is further expanding its production capacity at the Wuxi base. The newly added mAb/DS dual-function production line (BCM2 L2) is expected to start operation in the fourth quarter of 2024. The DP3 biologics conjugation production line is currently under construction and is expected to start operation in the second quarter of 2025.

Meanwhile, the production base of the Singapore group in Singapore has started construction in March 2024 and is progressing smoothly. It is expected to start operation at the end of 2025 or the beginning of 2026, with a capacity of 8 million bottles of conjugated biologics.
The Biosafety Law has not had a significant impact, and there is currently no evidence of order transfers.
Regarding the impact of the Biosafety Law, the company stated during the conference call that there is currently no evidence of customers switching orders due to this reason. The company is also continuously communicating with customers to keep them informed of the dynamic changes of the event.
Based on current observations, the number of new signed orders in the first half of the year is still increasing rapidly, especially in the second quarter, which is the first full quarter after the introduction of the Biosafety Law. The high growth of new signed orders confirms that the current impact is minimal.
According to the company's announcement, the group has completed over 110 GMP audits from global customers in the first half of the year, including 11 audits from European Union Qualified Persons (EU QPs).
In addition, the company's Wuxi base has also achieved a 100% success rate in global customer delivery. With high-quality production capacity and reliable delivery, it will become the foundation for the company to continue to acquire customers in the context of the legislation.
The performance of Pharmalease reflects not only significant benefits brought by the overall growth of the industry, but also key operational indicators that demonstrate a trend superior to the industry average. Despite the ongoing concerns in the market about the external environment, based on the company's actual performance, excessive pessimism seems no longer justified.

