8月23日,益诺思(688710.SH)开启申购,发行价格为19.06元/股,申购上限为0.8万股,市盈率15.4倍,属于上交所科创板,海通证券为保荐人及主承销商。
智通财经APP获悉,8月23日,益诺思(688710.SH)开启申购,发行价格为19.06元/股,申购上限为0.8万股,市盈率15.4倍,属于上交所科创板,海通证券为保荐人及主承销商。
招股书显示,益诺思是一家专业提供生物医药非临床研究服务为主的综合研发服务(CRO)企业,作为国内最早同时具备 NMPA 的 GLP 认证、OECD 的 GLP 认证、通过美国 FDA 的 GLP 检查的企业之一,与国际标准接轨,具备了行业内具有竞争力的国际化服务能力,为全球的医药企业和科研机构提供全方位的符合国内及国际申报标准的新药研究服务。
 公司服务主要涵盖生物医药早期成药性评价、非临床研究以及临床检测及转化研究三大板块,其中非临床研究板块具体包括非临床安全性评价、非临床药代动力学研究、非临床药效学研究。
公司服务主要涵盖生物医药早期成药性评价、非临床研究以及临床检测及转化研究三大板块,其中非临床研究板块具体包括非临床安全性评价、非临床药代动力学研究、非临床药效学研究。
经过多年的发展与积累,益诺思在国内非临床安全性评价细分领域市场占有率排名前三,处于行业领先地位。截至 2023 年 12 月 31 日,益诺思已拥有近 6 万平方米的现代化设施,以及一支业务精湛、综合素质高的研究队伍。报告期内,益诺思每年 90%以上收入均来自 I 类创新药物的非临床研究服务,已为客户完成了多个国际、国内首个创新药的研究服务。
自设立以来,益诺思凭借自身的前瞻性布局、创新性能力,逐步形成了重要靶器官毒性生物标志物评价技术、特殊毒性安全性评价关键技术、创新药物非临床安全性评价体系、实验动物特殊给药技术、放射性同位素标记与 Micro-PET/MR 影像技术、小核酸/多肽/ADC/CGT 产品生物分析技术平台、高灵敏度大分子多抗分析平台、流式技术受体占位分析平台等核心技术。
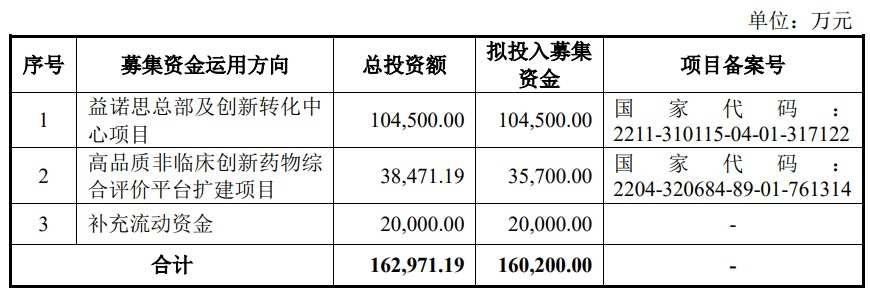
募集资金总额扣除发行费用后,拟用于募投项目情况如下:
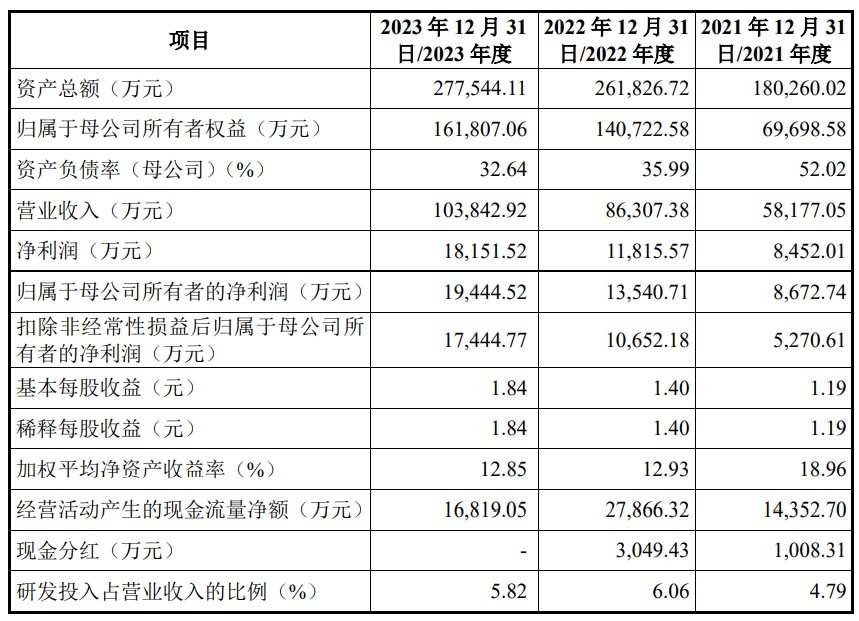
财务方面,2021年、2022年、2023年,益诺思实现营业收入分别约为人民币5.82亿元、8.63亿元、10.38亿元,期内净利润分别约为8452.01万元、1.18亿元、1.82亿元。