The basic fundamentals of Wuxi Bio remain stable for long-term development, and the core logic remains unchanged.
The number of new comprehensive projects has reached 61, which is not easy to achieve in the first half of this year in the face of many external uncertainties, exceeding market expectations. Although the income in the first half of the year increased by only 1% year-on-year, considering the high performance base of Wuxi Bio and the challenging development environment, the market expects stable growth rather than high growth. Wuxi Bio's steady growth performance proves the reliability of its "follow and win molecules" strategy.
From external factors, the expectation of a "rate cut" by the US Federal Reserve has increased significantly this year, and the global liquidity brought about by it has gradually improved the data of biomedical primary investment and financing. The rebound in downstream business cycles has brought considerable new orders to leading CXO companies such as Wuxi Bio (02269). This also confirms a view recognized by many investors at present: "As long as the water source is plentiful, there will be continuous growth logic for the CXO industry."
By deconstructing the key financial data disclosed in the 2024 interim report, investors can easily find that Wuxi Bio's mature back-end commercialization projects, abundant unfinished order volume, and considerable new projects are strong support for achieving stable growth in an adverse environment, demonstrating the company's strong internal growth capacity and risk resistance.
The basic fundamentals remain stable, and internal growth is expected.
Innovative pharmaceutical companies both at home and abroad have a strong demand for controlling research and development costs after experiencing a "financing winter", and they are more inclined to choose service providers like Wuxi Bio that offer high cost-effectiveness end-to-end services from a budget perspective. This is the core logic behind Wuxi Bio's ability to maintain a stable basic foundation despite constant external interference in the first half of this year.
According to the latest 2024 interim financial report, excluding the impact of the COVID-19 business, the company's revenue still achieved steady growth of 7.7% year-on-year, reaching 8.57 billion yuan, in line with its initial growth expectations of 5-10%. Among them, the income from non-COVID-19 late-stage clinical and commercial production projects increased by 11.7% year-on-year. At the same time, the company's net income for the period after adjustment was 2.25 billion yuan, reflecting its strong internal growth capacity and risk resistance in the face of various uncertainties.
From key financial indicators, it can be seen that Wuxi Biologics currently has a solid fundamental, which also indirectly confirms the important role of the company's CRDMO business model and the "follow and win molecule" strategy in promoting long-term sustainability.
In terms of business growth, during the reporting period, driven by the promotion of early research and development business, the company's project quantity achieved rapid growth. In the first half of 2024, the company signed 61 comprehensive projects, compared to 46 comprehensive projects added in the same period of the first half of 2023, making this the company's best performance in a half year so far. This also brings the number of ongoing comprehensive projects in the company to 742, making it one of the largest pipelines in the industry, covering a variety of complex biopharmaceutical products. It is not difficult to understand the importance of having a rich biopharmaceutical pipeline, as ADC, bispecific antibodies, monoclonal antibodies, fusion proteins, and other forms of drug molecules are currently popular. Although the high risk of drug development is widely known, there are always opportunities in a rich project pipeline to develop several billion-dollar-level drugs.
Looking closer, Wuxi Biologics currently has 359 pre-clinical projects, a year-on-year increase of 25.52%. Early-stage (Phase I and II) clinical development projects reached 311 (224 Phase I projects and 87 Phase II projects), a year-on-year increase of 15.6%. Late-stage (Phase III) clinical development projects and commercial production projects increased to 72 (56 late-stage projects and 16 commercial production projects), a year-on-year increase of 9%.
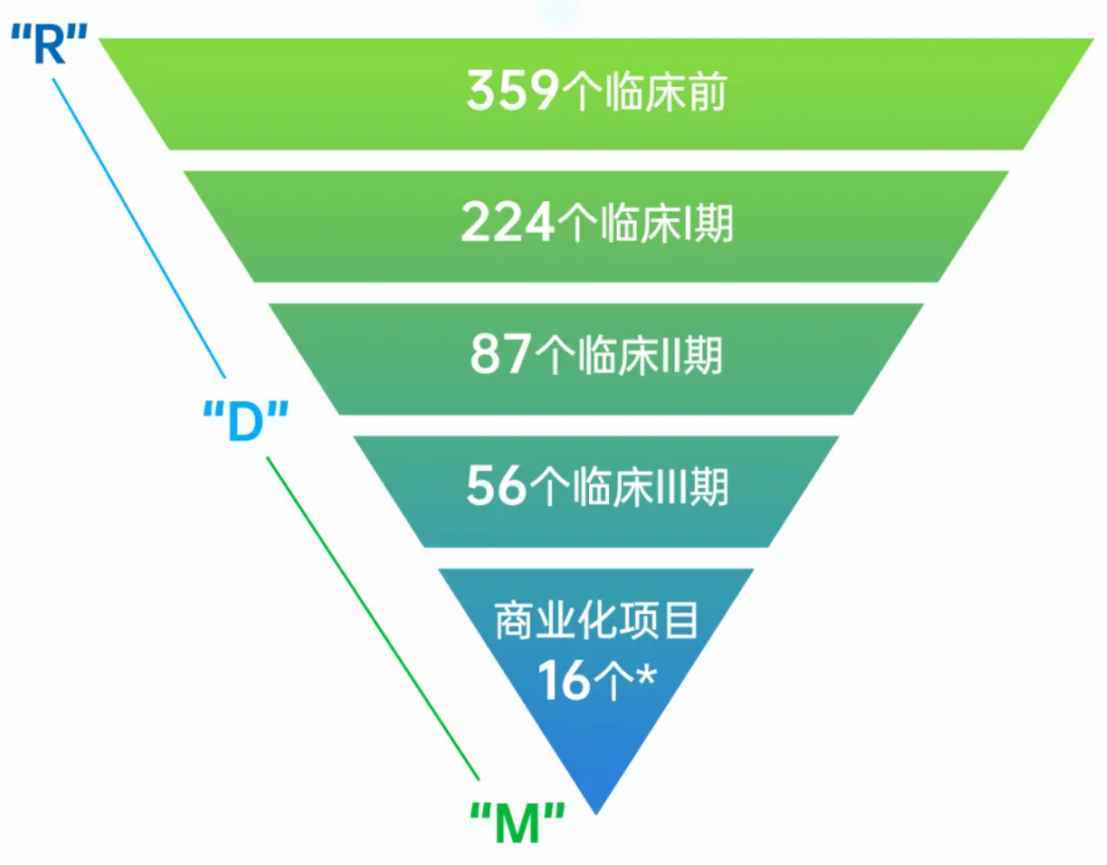
One prominent feature of Wuxi Biologics' project structure change in the first half of this year is that its pre-IND business growth is significantly higher than other sectors. This is closely related to the current downstream trends in the biopharmaceutical market.
According to information obtained from the Wise Finance app, HSBC Innovation Bank recently released a report pointing out that in 2023, there were only 161 financing deals totaling $4 billion in the global biopharmaceutical sector. However, in 2024 alone, there have been 71 financing deals totaling over $5.2 billion. The report also states that "whether it is exit or financing quantity, the first half of 2024 has already caught up with the full year of 2023 and is expected to reach the level of 2022."
As we all know, a healthy exit mechanism is an important indicator of the health of the biotechnology industry, and it indicates that the global biopharmaceutical industry is gradually recovering.
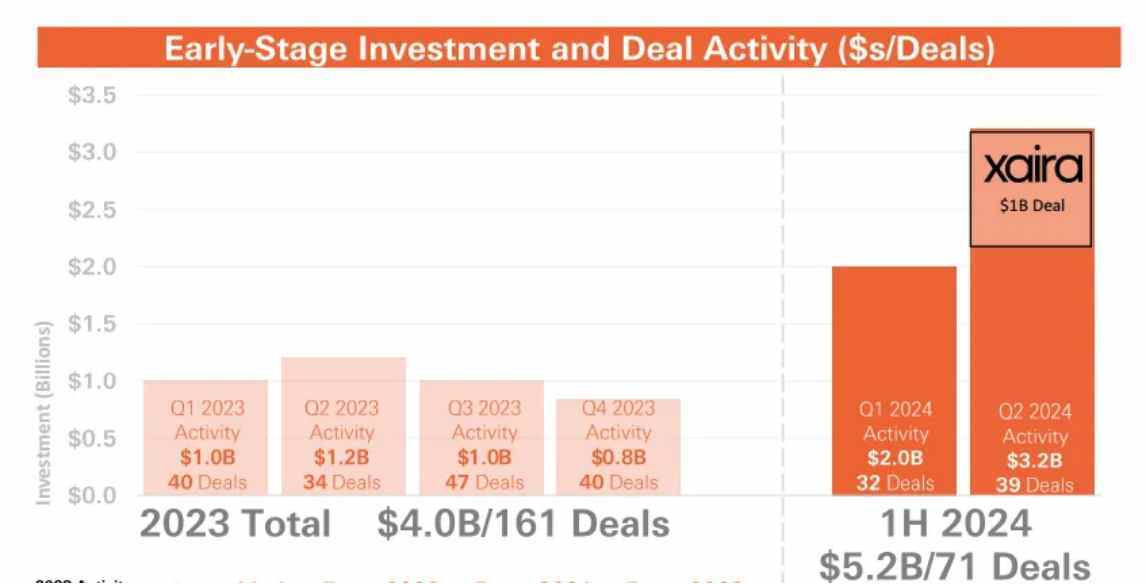
According to the "2024 US Biopharmaceutical Trading and Investment Report" recently issued by JPMorgan, the biopharmaceutical industry in the USA showed signs of recovery in the first half of 2024, with the second quarter being particularly active. Large funding rounds drove investment in biopharmaceutical and platform research, regardless of the clinical stage of the companies. In addition, biologics and small molecules continue to lead in early-stage investment and licensing transactions.
The rapid growth of early-stage projects also confirms the main logic of innovative pharmaceutical companies choosing "cost-effective" service providers based on cost control, as mentioned earlier.
Therefore, having sufficient order backlog is the main reason for the risk resistance and endogenous growth of top CXO companies like Wuxi Biologics.
Data shows that as of the end of June 2024, the total amount of Wuxi Biologics' unfinished orders reached $20.1 billion, which is basically the same as the first half of last year. Among them, the total value of unfinished service orders and potential milestone payment orders reached $13 billion and $7.1 billion, respectively. Over the next three years, the total value of unfinished orders will exceed $3.6 billion.
Furthermore, CEO Chen Zhisheng said at the interim performance communication meeting, "The total amount of unfinished orders mainly reflects the M-side. If we consider the R-side and D-side, the company's order income is actually very considerable." This is enough to show that the order backlog data guarantees the stable growth of the company's medium and long-term performance, and demonstrates its strong risk resistance and endogenous growth certainty.
Continued efforts in the R-side provide certainty in terms of services and technology.
At the performance communication meeting, CEO Chen Zhisheng also said, "In the current noisy environment, we need to see certainty. The company's performance in the first half of 2024 remains strong, thanks to our unique and efficient CRDMO business model and the successful execution of our 'follow and win molecules' global strategy."
From the perspective of business sectors, unlike most CMOs where the threshold for the M-side (drug production) business is relatively lower, Wuxi Biologics mainly focuses on the R-side (drug discovery) and D-side (drug development). The strong performance in the R-side business in the first half of this year is an important reason why Wuxi Biologics has exhibited strong performance resilience.
According to the Zhītōng Finance and Economics APP, WuXi Biologics reached licensing and research service agreements with multinational pharmaceutical companies GSK and BioNTech in the first half of last year, receiving initial payments of $40 million and $20 million, respectively.
In addition to direct collaboration with multinational pharmaceutical companies, WuXi Biologics is also using technology to empower its clients and help them accelerate their expansion into international markets. Recently, Merck announced its final agreement with SynCore Bio, acquiring the global rights to the novel bispecific antibody CN201 for the treatment of B-cell-related diseases through its subsidiary, with a consideration of $700 million as an initial payment and $600 million as milestone payment.
It is worth noting that CN201 was developed using WuXi Biologics' exclusive patent technology platforms, namely WuXiBodyTM, TCE, and WuXiUPTM. Its acquisition by multinational pharmaceutical companies with high initial and milestone payments reflects their high recognition of WuXi Biologics' research and development capabilities.
As mentioned above, both domestic and international innovative pharmaceutical companies have a strong demand for controlling R&D costs after experiencing a financing winter. WuXi Biologics stands out as an important choice for its exclusive and efficient technology platforms and reliable quality management system.
In terms of technology platforms, WuXiBodyTM, as a bispecific platform, can save 6 to 18 months of development time for each project compared to existing bispecific technology platforms, significantly reducing production costs. The company's WuXiUITM bioprocess technology patent platform, launched last year, can increase the yield of different molecular types, such as monoclonal antibodies, bispecific antibodies, and recombinant proteins, by 3 to 6 times within the same cultivation time, while reducing the production cost of the original solution in single-use bioreactors by 60-80% compared to traditional fed-batch cell culture processes.
The future development of the biomedical industry is not only about innovation, but also about the efficiency of innovation. WuXi Biologics plays a crucial role in this regard and should not be underestimated.
In terms of quality management, WuXi Biologics has undergone 21 inspections by EMA and FDA, with a 100% success rate in Pre-Approval Inspection (PAI). Since 2023, 14 client projects have successfully passed EMA inspections and 4 have passed FDA inspections, indicating the continuous recognition of WuXi Biologics' global quality system by authoritative regulatory agencies. Quality is the lifeline of drug research and production. Recently, there have been frequent news reports of large pharmaceutical companies experiencing production quality issues that affect the normal approval of their drugs. This further highlights the importance of having production partners with reliable quality systems.
The continuous expansion of production capacity at WuXi Biologics is a reflection of its advanced technology and reliable quality management system on the production side.
According to the Zhitong Finance APP, WuXi Biologics is enhancing its global layout and capacity building. Currently, WuXi Biologics' Ireland base is close to full production by 2025. Among them, MFG6.1 successfully completed its first process validation (PPQ) in the first half of this year, while the expansion plan for MFG6.2 is expected to be completed in the fourth quarter of this year. At the same time, MFG7 has also started commercial production. The company expects the Ireland base to enter stable operation stage by 2026. The integrated CRDMO center in Singapore is also officially entering the construction phase, with planned capacity of 0.12 million liters.
In summary, with leading platform technology and widely recognized international quality system support, WuXi Biologics' unique integrated model and the strategy of "following and winning molecules" continue to gain momentum, and the successful implementation of the dual-drive strategy will also continue to expand the differentiated advantage of the company with global leaders.
Combining the growing global CRDMO industry development space, the company's strong competitive barriers and outstanding order backlog, WuXi Biologics' inherent value is expected to continue to be released, and market valuation is expected to steadily rise.
