On the morning of August 26, Hong Kong Innovative Medical Equipment Company, Peijia Medical (9996.HK), held its 2024 interim performance conference to provide a detailed explanation of the company's interim performance.
The following are the key points of Peijia Medical's 2024 interim performance:
Steady revenue growth, the turning point from losses to profits in the divine introduction business
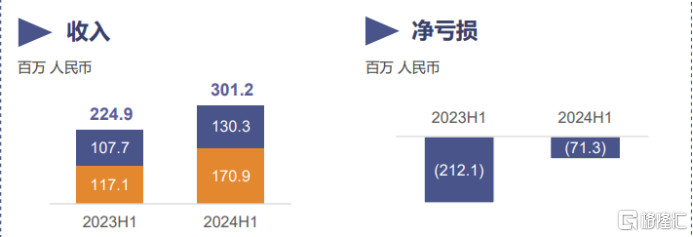
 Financial data shows that in the face of a complex market environment and the dual challenges of changing policies, the company achieved a revenue of approximately 30.1 billion yuan (RMB) in the first half of the year, a year-on-year increase of 33.9%. The revenue has shown a steady growth trend, further consolidating its leading position in the industry and laying a solid foundation for future development.
Financial data shows that in the face of a complex market environment and the dual challenges of changing policies, the company achieved a revenue of approximately 30.1 billion yuan (RMB) in the first half of the year, a year-on-year increase of 33.9%. The revenue has shown a steady growth trend, further consolidating its leading position in the industry and laying a solid foundation for future development.
At the same time, the company's first-half loss has narrowed significantly by 66.4% to around 71 million yuan compared to the same period last year. It can be seen that the company's financial situation has significantly improved, and the turning point to profitability is approaching, further boosting the confidence of investors and the market.
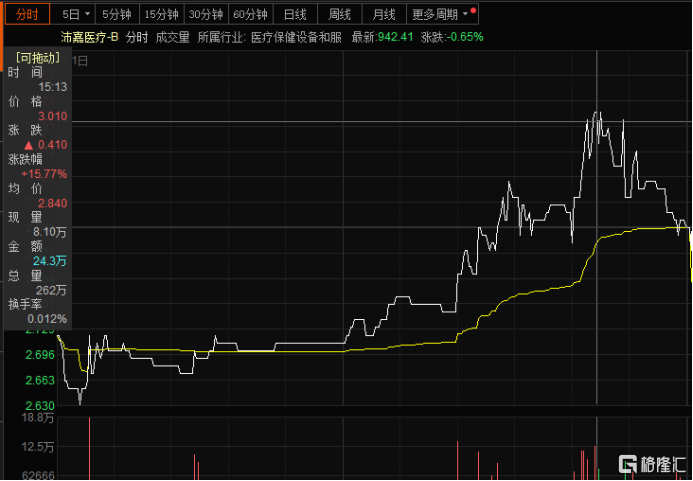
In terms of capital market performance, on the afternoon of August 26, the company's stock price rose by nearly 16%, demonstrating the market's confidence in its future prospects.
Analysis of revenue structure shows that the company's performance growth is mainly driven by the strong performance of two core sectors: transcatheter valve therapy ("valve") and neurointervention ("neuro"). The synergistic efforts of these two business sectors not only contribute to the steady growth of revenue for the company, but also further solidify the leading position of Peijia Medical in the market.
Valve business performance in the first half of the year was stable, with business revenue reaching 0.13 billion yuan, a year-on-year increase of 21.0%. From a product perspective, Peijia Medical's first and second generation TAVR products have experienced rapid growth in hospital admissions and market share. In the first half of the year, the company's products were used in nearly 100 new hospitals, with a cumulative coverage of over 580 hospitals. The terminal implantation of TAVR products reached nearly 1750 units, with a year-on-year growth rate of nearly 40%. The market share in the Chinese transarterial valve replacement (TAVR) market is approaching 25%, an increase of about 5 percentage points from the end of 2023, demonstrating the company's strong competitiveness in the transcatheter valve therapy market.
In terms of valve research and development pipeline, the company accelerated the progress of self-developed and BD pipelines in the first half of the year, and built a product portfolio and lineup with long-term competitiveness. The company's three strategic products, TaurusTrio system, TaurusNXT, and GeminiOne, have completed enrollment of patients in registered clinical trials. Based on the first and second generation products, AV21 small-sized valve was added to the product line. In addition, a new generation (2.5 generation) TAVR product, TaurusMax, which upgraded the valve imaging point and adjustability of the delivery system based on the second generation product TaurusElite, was approved for market release by the National Medical Products Administration in August. The rich product pipeline combined with price differentiation can meet the diversified market demand and provide a powerful engine for the company's future performance growth, further enhancing its competitiveness in the market.
In the first half of the year, the neurointervention business performed exceptionally well, with segment revenue reaching 0.171 billion yuan, achieving a rapid growth of 45.9%. The neurointervention business segment turned losses into profits, with a profit of 28.716 million yuan, far exceeding the initial guidance.
The rapid popularization and penetration of domestic neurointerventional surgeries have brought significant sales growth for the company's bleeding, ischemia, and access-related products. The full-line products of the neurointervention business have achieved remarkable performance. The implementation of centralized procurement policies has driven the rapid increase in sales of spring coils in both in-market and out-of-market segments, with strong revenue growth. Ischemic products such as Syphonet clot retrieval stent and Fastunnel delivery balloon dilatation catheter have rapidly increased market penetration rate due to their differentiated design features and the application of innovative techniques developed in collaboration with surgeons. In addition, DCwire micro guide wire, a access-related product approved by the National Medical Products Administration in mid-2023, has gained deep brand trust among surgeons and patients due to its excellent product performance, contributing millions of revenue to the company.


Significant improvement in operational efficiency.
With the increasingly mature operation system, the operational efficiency of Peijia Medical has been significantly improved. In the first half of the year, the expense ratio in sales, management, and research and development all achieved significant reductions. From the group's perspective, the sales expense ratio, management expense ratio, and R&D expense ratio decreased by 26.2, 7.0, and 42.8 percentage points year-on-year respectively.
Looking at the valve business, the sales expenses decreased by 14.0% year-on-year, and the sales expense ratio decreased by 34.0 percentage points to 83.7%. The per capita output (efficiency) of the valve business sales team has increased from one million yuan in 2023 to one hundred and forty million yuan, an increase of nearly 40% year-on-year. Effective control of sales expenses not only enhances the company's profitability, but also enhances its market competitiveness. At this point, the commercial loss of the valve business has significantly reduced by 92.4% to 2.505 million yuan, approaching commercial profitability. This undoubtedly proves the strong execution and excellent market performance of Peijia Medical.
In addition, Peijia Medical continues to improve operational efficiency through measures such as optimizing production processes and increasing production efficiency. The construction of the new headquarters and the increase in production capacity have provided strong support for the rapid growth of the company's business. At the same time, the company has also reduced the production cost of valves and improved the security of the supply chain by introducing more key raw material providers and optimizing the internal manufacturing process of self-produced raw materials.
Looking at the hemodialysis business, the sales expense ratio, management expense ratio, and R&D expense ratio all decreased by 13.8, 4.0, and 4.8 percentage points year-on-year respectively. While achieving a turnaround from loss to profit in the branch, operational efficiency has improved.
In terms of financial reserves, Peijia Medical has also shown stable performance. In the first half of the year, the net cash used in operating activities decreased significantly by 91.2% year-on-year to 41.4 million yuan, and cash, cash equivalents, and term deposits were approximately 0.83 billion yuan. With the completion of the registration and enrollment of patients for the three major products in the first half of the year and the reduction of remaining milestone payments for BD to three products (Edward Lifesciences' acquisition of JenaValve, exemption of remaining milestone payments for the reflux valve project TaurusTrioTM/TrilogyTM), the major spending phase of Peijia Medical's R&D projects is nearing completion. Through comprehensive improvement in operational efficiency, the company has not only optimized the operating environment, but also laid a solid foundation for future sustained and stable development. Overall, Peijia Medical's cash reserves can support daily operations until overall profitability.
Looking ahead, with the continuous optimization and improvement of Peijia Medical's product development, commercialization expansion, and operational efficiency in the valve and hemodialysis businesses, Peijia Medical is expected to achieve more stable, robust, and sustainable development in the future. At the same time, the company will continue to uphold the concept of "Excellence in Heart, Reverence for Life", and continue to focus on providing higher quality and more efficient medical solutions in the valve and hemodialysis field for patients worldwide.
The following is a partial record of the Q&A session between investors and management at the earnings conference:
Q1: How do you view Edwards' investment strategy? What is the potential impact on Peijia Medical?
In terms of Pega Medical, Edward's investment strategy shows a focus on the aortic regurgitation (AR) field, and the company's clinical data has also been helpful in Edward's decision-making. This is a positive signal for the company, and the registration of clinical patients for the regurgitation valve has exceeded expectations in the first half of the year and has entered the follow-up stage. We look forward to the regurgitation valve of the company bringing greater clinical benefits to patients.
Q2: What is the expected timeline for Pega Medical to reduce losses and achieve profitability?
A2: As a platform company, profitability is the goal rather than the means. The purpose of the company's listing financing is for long-term development rather than short-term cashing out. Currently, Pega Medical's focus is on product research and development and commercial layout, and significant growth is expected in the next few years. Although achieving profitability in the short term is challenging, the company has taken multiple measures to reduce losses, and the transcatheter business has turned from loss to profit in the first half of the year. This year, the company has significantly reduced losses and plans to continue efforts to reduce losses next year in order to achieve a balance between profit and loss. The company expects to achieve overall profitability in 2026.
Q3: What is the pricing strategy for the company's 2.5th generation products? What are its market advantages?
A3: The company's 2.5th generation products are positioned in the high-end market and will be priced higher than existing products. This product has the most advanced dual-axis adjustable bending function in the world, which can solve the pain points in the clinical surgical treatment process and improve the success rate of the surgery. These advantages will make the 2.5th generation product highly competitive in the market. The company has three valve registration certificates, which can help cover a deep market with different payment capabilities and diverse clinical needs.
Q4: What specific measures and results does Pega Medical have in cost control?
A4: The company improves communication, execution, and decision-making efficiency through digital operation. In terms of the supply chain, the company continuously optimizes the process flow in the production process to improve production efficiency. In the future, the company will continue to focus on the market competition pattern and the trend of cost changes to further optimize cost control measures.
Q5: With the launch of the 2.5th generation product, will the sales expenses increase?
Regarding the sales expenses, a significant portion is allocated to personnel salaries, while market expenses are relatively fixed. For new products, they will not have much impact and are unrelated to the quantity of products. In terms of proportion, as sales increase, the sales expense ratio will decrease. The promotion of new products will mainly utilize the existing team, as we have already covered mainstream academic and market activities, which will have a synergistic effect and prevent significant cost increase.
Q6: What are Peijia Medical's expectations for future sales and research and development (R&D) expenses?
A6: As the company's product line continues to improve and market share gradually expands, sales expenses will remain relatively stable. Fixed components in management and sales expenses, such as personnel salaries and conference expenditures, will decrease relative to income growth. As for R&D expenses, the company will allocate resources based on the progress and requirements of R&D projects. Although R&D expenses may increase in the short term, in the long run, successful commercialization of projects will generate significant returns. Therefore, the company maintains an optimistic outlook for future sales and R&D expenses.
Q7: What are Peijia Medical's upcoming new products for the IPO? How are these products expected to impact revenue?
A7: Peijia Medical's new products include reflux valves and long-term valves, among other heavyweight products, which are expected to be launched gradually within the next two years. These products all have high market potential and competitive advantages, can meet diversified market demands, and are expected to quickly capture market share and become new growth drivers for the company. They are expected to have a significant positive impact on the company's overall revenue.
Q8: Does Peijia Medical have a focus on the bleeding and ischemic pathways in neuro intervention?
A8: The management believes that all three product lines are equally important. Currently, the company has four registered certificates for coil springs, one auxiliary bracket, and one cooperative product that is about to be certified. All bleeding product lines have been completed. The next step is to leverage the high penetration rate and smooth market acceptance of coil springs, which are highly recognized by customers, to drive the brackets. In terms of ischemia, there are two aspects: emergency thrombectomy, where the combination of products such as Fluxcap balloon catheter guide and Tethys AS aspiration catheter form the emergency thrombectomy product portfolio, and Syphonet thrombectomy bracket, which has surpassed all domestic companies in performance after being on the market for two years. Upgrades and replacements will also be made to the product line. The other aspect is narrowness, which includes balloon-based products. The company is the second domestic company in the balloon business and has a good basis for market entry. The Fastunnel delivery balloon catheter, as a unique product in the market, has quickly gained customer recognition, reducing surgical steps and improving surgical safety. NeuroStellar narrow treatment bracket has submitted all clinical data in August and is currently in the registration stage. There are already products that combine dilation and implantation, and future layout will continue. The pathway is a large category with many products having a solid foundation in operations, and the learning curve is very short. Once doctors feel that the product has a good feel, they will quickly use it. DCwire has gained great recognition from customers, boosting the overall revenue of guidewires. There are quite a number of pathway products, and two pathway products will be submitted for approval this year. In the field of neuro intervention, the company's competitiveness can only be enhanced if all product lines progress simultaneously, providing support in various areas.
Q9: How is Peijia Medical progressing with the mitral valve replacement project in China? What are the company's plans for research and development in the United States?
A9: The company will invite foreign teachers to promote the mitral valve replacement project in China, which has been steadily progressing and has made significant progress. Globally, mitral valve replacement is the only project not yet conquered in the field of valve technology, at the forefront of global technology and the focus of the company. The company has done nearly 20 cases, and the results are very good. Currently, the biggest difficulty is finding suitable patients, but the company is still determined to promote the project. At the same time, the company's tricuspid valve replacement project in the United States is also progressing smoothly and has received support from internationally renowned teams. In the future, the company will continue to increase research and development investment in the field of mitral and tricuspid valve replacement and plans to complete multiple important clinical trials and registration applications in the next few years to promote the successful commercialization of the project.
Q10: What is the clinical significance of tricuspid valve replacement? What market opportunities does Peijia Medical have in this field?
A10: In the past, the tricuspid valve was neglected, but later, with Edwards' entry, more doctors and patients began to pay attention to this disease. The tricuspid valve replacement field has important clinical significance and involves the treatment needs of a large number of heart disease patients. It is currently the hottest topic overseas. With the advancement of medical technology and the trend of population aging, the demand for the treatment of tricuspid valve disease will continue to grow. The company's products currently have a good response overseas and are currently in the forefront of research and development. The company also hopes to achieve internationalization.
Note:
[1] Commercial loss refers to gross profit minus sales and distribution expenses.

 财报数据显示,在面临复杂市场环境以及多变政策的双重挑战下,公司上半年实现营收约3.01亿元(人民币,下同),同比增长33.9%。营收呈稳健增长态势,进一步巩固了其在行业内的领先地位,为未来的发展奠定了坚实的基础。
财报数据显示,在面临复杂市场环境以及多变政策的双重挑战下,公司上半年实现营收约3.01亿元(人民币,下同),同比增长33.9%。营收呈稳健增长态势,进一步巩固了其在行业内的领先地位,为未来的发展奠定了坚实的基础。