①复星医药上半年创新药收入超37亿元,约占整体营收的18%。吴以芳透露,创新药业务在未来几年将争取达到30%的年复合增长率。②吴以芳表示,复宏汉霖的私有化正在推进之中,将通过私有化,把复宏汉霖的整个业务、管线和复星医药深度融合起来。
《科创板日报》8月29日讯(记者 郑炳巽)“去年年底,由新冠带来的压力已经出清了,今年上半年我们开始进入一个恢复性增长的阶段。”在28日举行的业绩交流会上,复星医药(600196.SH)董事长吴以芳说道。
2024年半年报显示,复星医药实现营收204.63亿元,同比下降4.36个百分点,归母净利润12.25亿元,同比下降31.09%。复星医药表示,若不含新冠相关产品同比下降的影响,上半年营收同比增长5.31%。
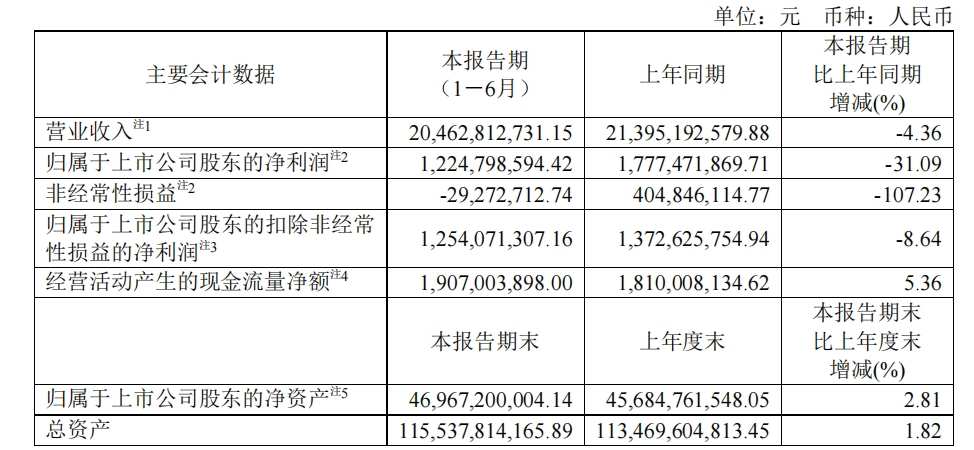
 目前,按不同业务板块划分,复星医药的营收主要来自制药、医疗器械与医学诊断、医疗健康服务,制药板块仍是复星医药最大收入来源,该板块报告期内实现营收146.77亿元,同比减少8.24%,占营收比重为71.72%。
目前,按不同业务板块划分,复星医药的营收主要来自制药、医疗器械与医学诊断、医疗健康服务,制药板块仍是复星医药最大收入来源,该板块报告期内实现营收146.77亿元,同比减少8.24%,占营收比重为71.72%。
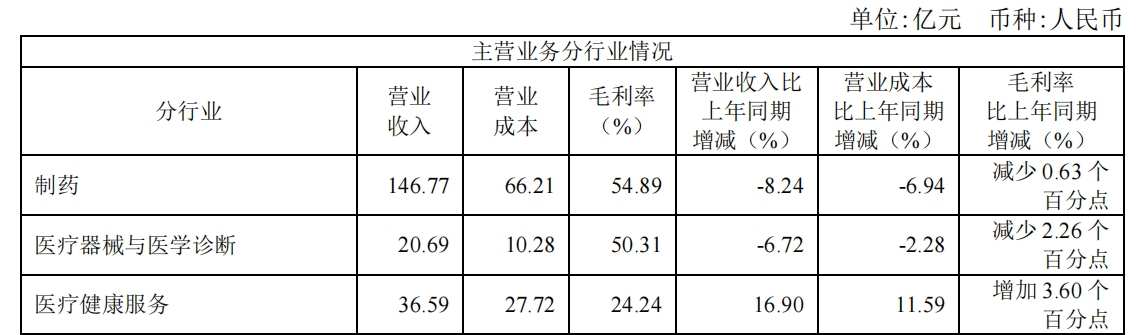
针对制药板块收入减少,吴以芳在业绩会上解释道,“一方面,集采和医保价格谈判对于制药板块的收入有一定影响,另一方面,去年年底参加医保谈判的创新药还在进院的过程之中,在一、二季度的销售仍较低。”吴以芳表示,创新药在三季度的增长将会提升,“整个创新药的收入增长,在未来几年将争取达到30%的年复合增长率。”
上半年,复星医药自研及许可引进的4个创新药/生物类似药一共有9项适应症于境内外获批,38个仿制药品种于境内外获批。其中,创新药收入超过37亿元,占整体营收比例约18%,占制药板块比例约25%。
“未来,常规仿制药大概还是保持一位数的增长,创新药在5年周期里估计会占到收入的一半,甚至更高。”吴以芳继续说道,“但我们还是会坚持仿、创结合,仿制药的盈利很大程度上支持着我们创新药的投入。”
在创新药收入方面,复星医药控股子公司复宏汉霖(2696.HK)起到了很大的贡献。
复宏汉霖上半年实现营收27.46亿元,同比增长约9.8%,净利润3.86亿元,同比增长约61.0%。5款产品的销售收入合计24.79亿元,同比增长15.2%。其中,注射用曲妥珠单抗(汉曲优)、斯鲁利单抗(汉斯状)、贝伐珠单抗(汉贝泰)分别实现销售收入14.74亿元、6.78亿元、0.87亿元。
值得一提的是,汉斯状是复星医药自研的首款生物创新药,也是全球首个用于一线治疗小细胞肺癌(SCLC)的抗PD-1单抗,从2022年国内获批上市以来,目前已覆盖四项适应症,第五项适应症一线治疗非鳞状非小细胞肺癌(nsNSCLC)的上市注册申请也已获国家药监局受理,有望今年下半年在国内获批准。
吴以芳透露,复星医药发展至今,打造两三亿元的品种对公司的贡献非常有限,“我们未来的重要任务,是着眼于真正的高价值临床产品,打造超10亿元的大品种。”他认为,包括汉斯状和汉曲优在内的多个产品,虽然还处于上市不久的拓展进程,但未来都有潜力成为10亿元及超过10亿元的大单品。
谈及复宏汉霖,吴以芳在业绩会上也透露了其私有化的相关进展,“复宏汉霖的私有化我们在正常推进之中。不管如何,我们的战略目标是非常清楚的,那就是通过私有化,把复宏汉霖的整个业务、管线和复星医药深度融合起来。”
“目前,复宏汉霖主要围绕ADC、单抗方向开发肿瘤药物,复星医药在肿瘤领域有更广泛的适应症。私有化之后和母公司结合,复宏汉霖在肿瘤领域的范围也会拓宽很多的。”
吴以芳还认为,私有化将帮助复宏汉霖更好实现“出海”,“在出海过程中,母公司有很多能力走在前面了,这些可以赋能复宏汉霖。同时,复宏汉霖也有很多产品具备国际化基础,与复星医药的出海业务有很好的协同。”
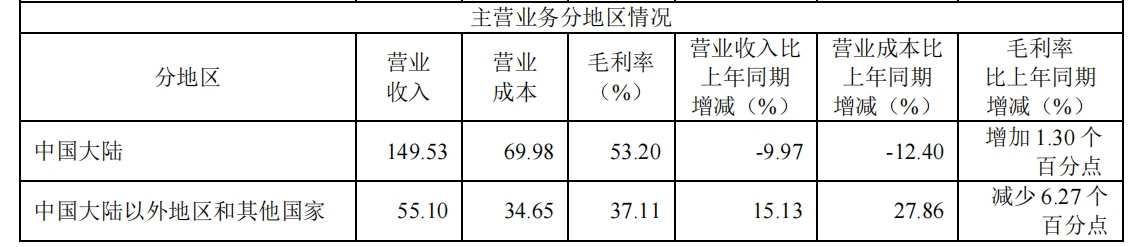
据悉,上半年复星医药来自海外的收入达55.10亿元,同比增长15.13%,占营收比例为26.93%,较去年同期提升4.56个百分点。目前,复星医药海外商业化团队近1000人,覆盖了美国、欧洲、非洲等海外市场。
具体到部分产品上,上半年,汉曲优有3项适应症获美国FDA批准上市,涵盖乳腺癌及胃癌领域,成为在中国、欧盟、美国三地获批的国产生物类似药。汉斯状也于2024年1月完成首批海外发货,成为首个在东南亚国家获批上市的国产PD-1单抗,其于欧盟的上市许可申请(MAA)亦已于2023年3月获得欧洲药品管理局(EMA)受理。
