In the third quarter, jiangsu hengrui pharmaceuticals' eps was 0.19 RMB, a year-on-year increase of 5.56%. In the first three quarters, jiangsu hengrui pharmaceuticals' profit increased by 32.98% year-on-year, with eps increasing by 32.73% year-on-year. The main reason is that the company recognized the upfront payment received from Merck Healthcare of 0.16 billion euros as revenue, which significantly increased the profit.
Jiangsu Hengrui Pharmaceuticals achieved steady growth in revenue and profit in the third quarter, with revenue reaching 6.589 billion RMB, a year-on-year increase of 12.72%. In the first three quarters, net income and EPS of Jiangsu Hengrui Pharmaceuticals both soared by over 30%, mainly due to the company recognizing the upfront payment of 0.16 billion euros received from Merck Healthcare as revenue, resulting in a significant increase in profit.
On Thursday, October 24, Jiangsu Hengrui Pharmaceuticals released its third-quarter financial report:
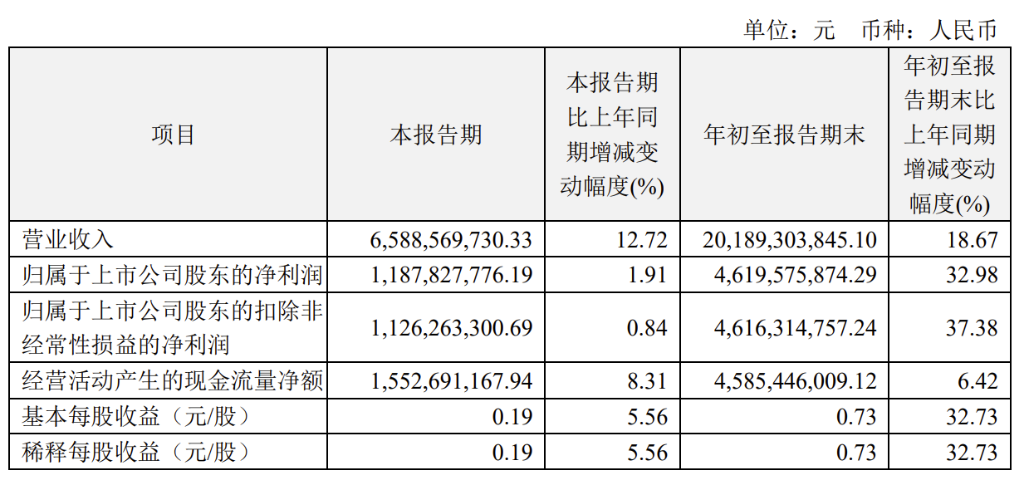
- Third-quarter revenue: 6.589 billion RMB, a year-on-year increase of 12.72%; Revenue for the first three quarters: 20.189 billion RMB, a year-on-year increase of 18.67%.
- Third-quarter net income: 1.188 billion RMB, a year-on-year increase of 1.91%; Net income for the first three quarters: 4.62 billion RMB, a year-on-year increase of 32.98%.
- Third-quarter EPS: 0.19 RMB, a year-on-year increase of 5.56%; EPS for the first three quarters: 0.73 RMB, a year-on-year increase of 32.73%.
- Net income excluding non-recurring gains and losses: 1.126 billion RMB, a year-on-year increase of 0.84%.
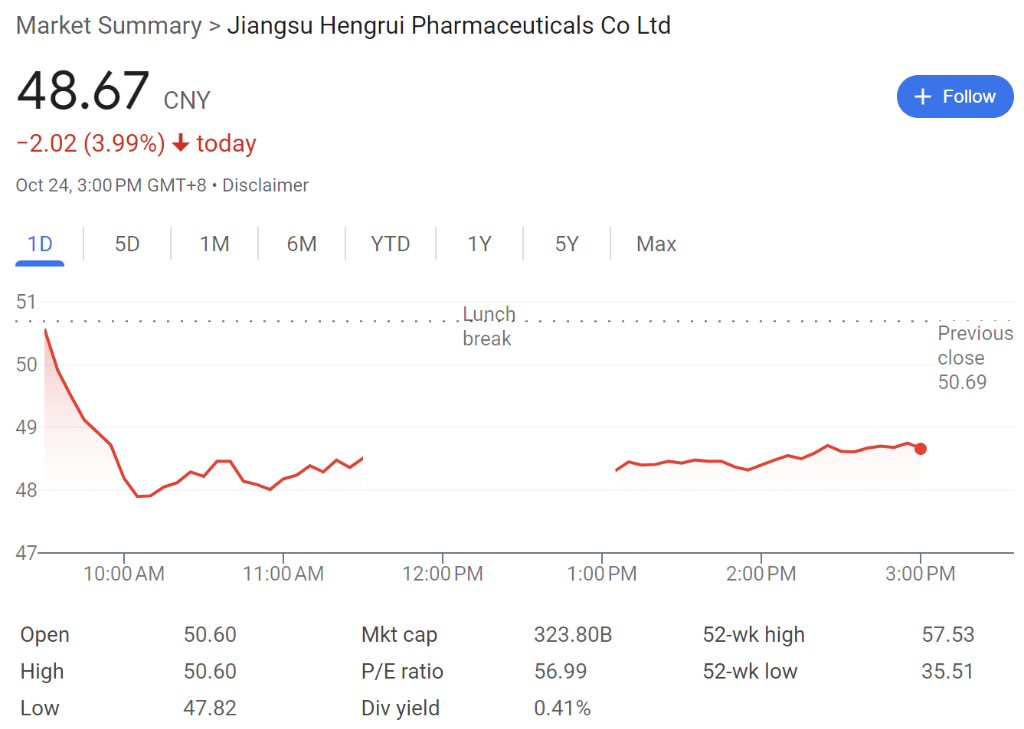
- Total assets as of the end of the third quarter: 48.338 billion RMB, compared to 43.785 billion RMB at the end of 2023.
 In the first three quarters, jiangsu hengrui pharmaceuticals' profit increased by 32.98% year-on-year, and earnings per share increased by 32.73% year-on-year. The main reason is that the company recognized the upfront payment of 0.16 billion euros received from Merck Healthcare as revenue, leading to a significant increase in profit.
In the first three quarters, jiangsu hengrui pharmaceuticals' profit increased by 32.98% year-on-year, and earnings per share increased by 32.73% year-on-year. The main reason is that the company recognized the upfront payment of 0.16 billion euros received from Merck Healthcare as revenue, leading to a significant increase in profit.
hengrui pharmaceuticals fell by 4% today to 48.67 RMB per share. Over the past year, hengrui pharmaceuticals has risen by more than 12%. As of the time of writing, hengrui pharmaceuticals has a market cap of 323.8 billion RMB.
jiangsu hengrui pharmaceuticals: vigorously investing in innovative research and development.
According to the financial report, jiangsu hengrui pharmaceuticals' research and development investment in the first three quarters was 4.549 billion RMB, a significant increase of 22% year-on-year. The substantial investment in research and development has brought rich results to jiangsu hengrui pharmaceuticals:
On October 15, jiangsu hengrui pharmaceuticals announced that its subsidiary, Beijing Shengdi Pharmaceuticals Co., Ltd., has been issued the 'Drug Clinical Trial Approval Notice' for the SHR-6934 injection by the National Medical Products Administration. The drug is intended for the treatment of heart failure, with cumulative R&D expenses of approximately 8.24 million RMB.
On October 10, jiangsu hengrui pharmaceuticals announced that the Injection Paclitaxel (Albumin-bound) ANDA filed with the FDA has been approved, becoming the first manufacturer to receive approval for this generic drug category in the USA. The drug is used to treat metastatic breast cancer that has failed combination chemotherapy, with cumulative R&D expenses of approximately 52.91 million RMB.
On October 9th, Jiangsu Hengrui Pharmaceuticals announced that it has received the approval from the National Medical Products Administration for the issuance of the "Drug Clinical Trial Approval Notice" for the injection HRS-2183. The indication applied for is the treatment of severe infections caused by gram-negative bacteria with limited or no treatment options. Clinical trials will be conducted in the near future, and the total accumulated research and development expenses for the project are approximately 19.35 million yuan.

 前三季度,恒瑞医药利润同比增长32.98%,每股收益同比增长32.73%,主要原因是公司将收到的Merck Healthcare 1.6亿欧元对外许可首付款确认为收入,利润增加较多。
前三季度,恒瑞医药利润同比增长32.98%,每股收益同比增长32.73%,主要原因是公司将收到的Merck Healthcare 1.6亿欧元对外许可首付款确认为收入,利润增加较多。