①由於帶狀皰疹減毒活疫苗銷量減少,百克生物第三季度實現營收同比下降40.37%,歸母淨利潤同比下降51.41%;②當前,國內除了GSK的帶狀皰疹疫苗與百克生物爭奪市場之外,還有超過10家企業正在開發同類產品,百克生物面臨多個潛在競爭對手。
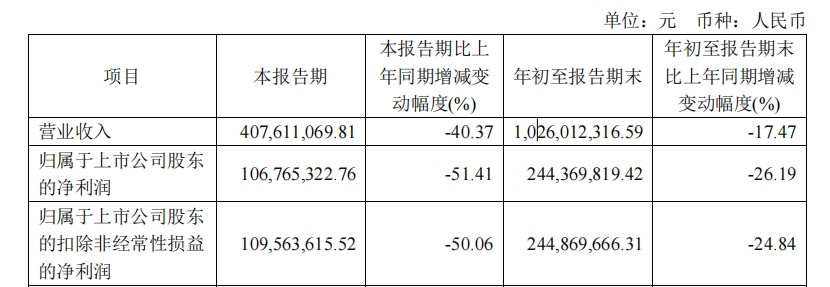
《科創板日報》10月25日訊(記者 鄭炳巽)25日晚間,知名疫苗公司百克生物(688276.SH)正式發佈2024年第三季度報告。報告期內,由於帶狀皰疹減毒活疫苗銷量減少,百克生物第三季度實現營收4.08億元,同比下降40.37%,歸母淨利潤1.07億元,同比下降51.41%。
受第三季度業績拖累,百克生物1-9月份累計實現營收10.26億元,同比下降17.47%,歸母淨利潤2.44億元,同比下降26.19%。若不含第三季度業績,百克生物上半年實現營收6.18億元,同比增長10.50%,歸母淨利潤1.38億元,同比增長23.54%。
 作爲一家專業從事人用疫苗研發、生產、銷售的企業,百克生物目前擁有3款已獲批上市的疫苗產品,包括水痘疫苗、鼻噴流感疫苗,以及帶狀皰疹疫苗。其中,帶狀皰疹疫苗在2023年年初獲得國家藥監局批准上市,百克生物也因此打破了國內帶狀皰疹預防產品由進口疫苗壟斷的局面。
作爲一家專業從事人用疫苗研發、生產、銷售的企業,百克生物目前擁有3款已獲批上市的疫苗產品,包括水痘疫苗、鼻噴流感疫苗,以及帶狀皰疹疫苗。其中,帶狀皰疹疫苗在2023年年初獲得國家藥監局批准上市,百克生物也因此打破了國內帶狀皰疹預防產品由進口疫苗壟斷的局面。
在帶狀皰疹疫苗上市之前,百克生物的最大收入來自於水痘疫苗。以2021年與2022年爲例,百克生物實現營收12.02億元、10.71億元,其中水痘疫苗的收入分別爲10.20億元、9.57億元,佔整體營收比例分別爲84.88%、89.33%。
隨着帶狀皰疹疫苗上市,百克生物2023年全年實現營收18.25億元,同比增長70.30%。其中,帶狀皰疹疫苗實現銷售收入8.83億元,佔整體收入的48.38%,超過水痘疫苗8.20億元的銷售收入及44.93%的收入佔比。
不過,百克生物此前也提醒,公司帶狀皰疹疫苗獲批上市不久,新產品市場培育需要一定時間,如市場準入、推廣等未達預期,市場增長空間可能會受到一定的限制。
在百克生物的產品上市之前,全球範圍內還有2款帶狀皰疹疫苗上市,分別爲默沙東的Zostavax和葛蘭素史克的Shingrix。
Zostavax雖然很早在2006年就獲得FDA批准上市,但未進入中國市場,而Shingrix在2017年獲FDA批准上市之後,也於2019年獲得國家藥監局批准進口註冊申請,並於2020年6月正式在中國上市銷售。當前,Shingrix無疑是百克生物帶狀皰疹疫苗的「勁敵」。
2023年,Shingrix實現銷售額34.46億英鎊,摺合約人民幣314.22億元,成爲葛蘭素史克銷售額最高的產品。中金公司研究部估計,2023年Shingrix在中國的銷售額約爲20億元。
值得一提的是,葛蘭素史克已經在2023年與智飛生物(300122.SZ)就Shingrix達成獨家合作協議,由智飛生物在中國大陸地區營銷、推廣、進口並銷售Shingrix。根據協議,2024-2026年間,Shingrix疫苗最低採購金額分別爲34.4億元、68.8億元、103.2億元,總計206.4億元。中金公司預計,Shingrix較2023年前放量將明顯提速。
事實上,在帶狀皰疹疫苗賽道上,除了已經上市的產品給百克生物帶來挑戰之外,還有多家企業正在佈局相關產品,百克生物正面臨多個潛在競爭對手。
截至2024年5月,全球範圍內有17家企業正在研發帶狀皰疹疫苗。其中,僅中國就佔了14家,進度靠前的有綠竹生物、怡道生物/中慧元通、邁科康生物等企業,相關產品處於III期臨床,其他已經開啓臨床研究的企業還有康希諾、瑞科生物等。
據《帶狀皰疹疫苗預防接種專家共識(2022年版)》,全球普通人群帶狀皰疹發病率爲每年1000人約3-5人,而中國每年這一數據爲1000人約2.8-5.8人。弗若斯特沙利文數據顯示,2021年,中國50歲及以上人群中新增帶狀皰疹病例數爲390萬,預計2025年將達到490萬。

 作为一家专业从事人用疫苗研发、生产、销售的企业,百克生物目前拥有3款已获批上市的疫苗产品,包括水痘疫苗、鼻喷流感疫苗,以及带状疱疹疫苗。其中,
作为一家专业从事人用疫苗研发、生产、销售的企业,百克生物目前拥有3款已获批上市的疫苗产品,包括水痘疫苗、鼻喷流感疫苗,以及带状疱疹疫苗。其中,