11月6日,君实生物(01877)宣布,针对顺铂不耐受的复发/转移性鼻咽癌(R/M NPC)患者的临床研究——特瑞普利单抗联合吉西他滨一线治疗顺铂不耐受的R/M NPC的2期研究最新数据在国际期刊《细胞报告医学》(Cell Reports Medicine)上发表。
智通财经APP获悉,11月6日,君实生物(01877)宣布,针对顺铂不耐受的复发/转移性鼻咽癌(R/M NPC)患者的临床研究——特瑞普利单抗联合吉西他滨一线治疗顺铂不耐受的R/M NPC的2期研究最新数据在国际期刊《细胞报告医学》(Cell Reports Medicine)上发表。
研究结果显示,患者客观缓解率(ORR)达61.9%,疾病控制率(DCR)为100%,中位无进展生存期(PFS)达11.8个月,且毒性较标准含铂治疗显著降低,仅23.8%的患者发生≥3级不良事件(AE)。君实生物新闻稿表示,该治疗方案为R/M NPC顺铂不耐受患者带来了疗效和安全性的全面优化和升级。
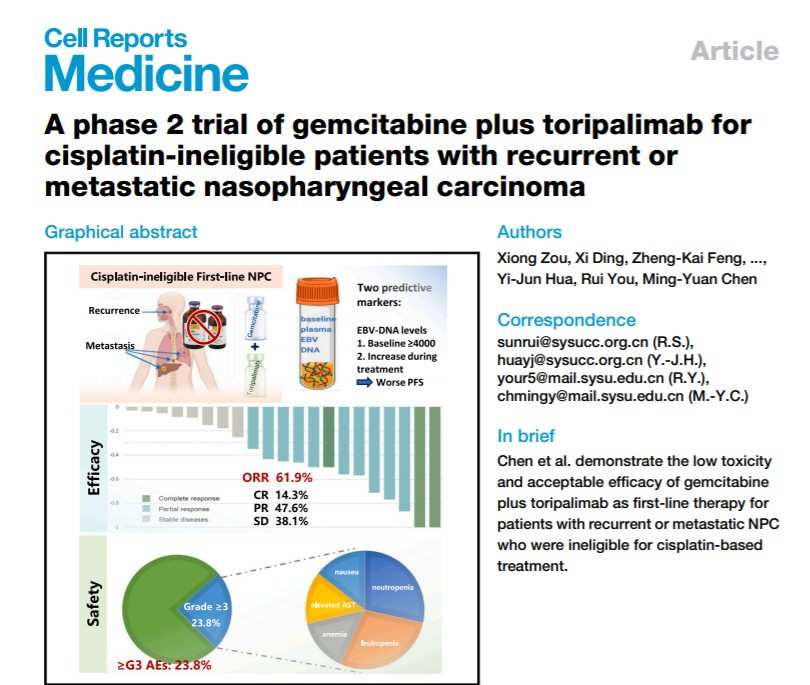
 以特瑞普利单抗为代表的抗PD-1单抗联合吉西他滨+顺铂(GP)方案已成为R/M NPC患者的一线标准治疗方案。然而顺铂毒性较大,存在部分患者由于肾功能受损、体力状况较差、听力丧失或心力衰竭等原因无法耐受顺铂治疗。特瑞普利单抗已经相继在中国、美国、欧盟获批用于治疗R/M NPC。考虑到长期疗效和低毒性特征,本研究假设特瑞普利单抗联合毒性较小的化疗药物(吉西他滨)有望在不降低疗效的前提下提高安全性,最终达到去铂目的。
以特瑞普利单抗为代表的抗PD-1单抗联合吉西他滨+顺铂(GP)方案已成为R/M NPC患者的一线标准治疗方案。然而顺铂毒性较大,存在部分患者由于肾功能受损、体力状况较差、听力丧失或心力衰竭等原因无法耐受顺铂治疗。特瑞普利单抗已经相继在中国、美国、欧盟获批用于治疗R/M NPC。考虑到长期疗效和低毒性特征,本研究假设特瑞普利单抗联合毒性较小的化疗药物(吉西他滨)有望在不降低疗效的前提下提高安全性,最终达到去铂目的。
