CISCO initiates the first clinical study of pan-KRAS inhibitors.
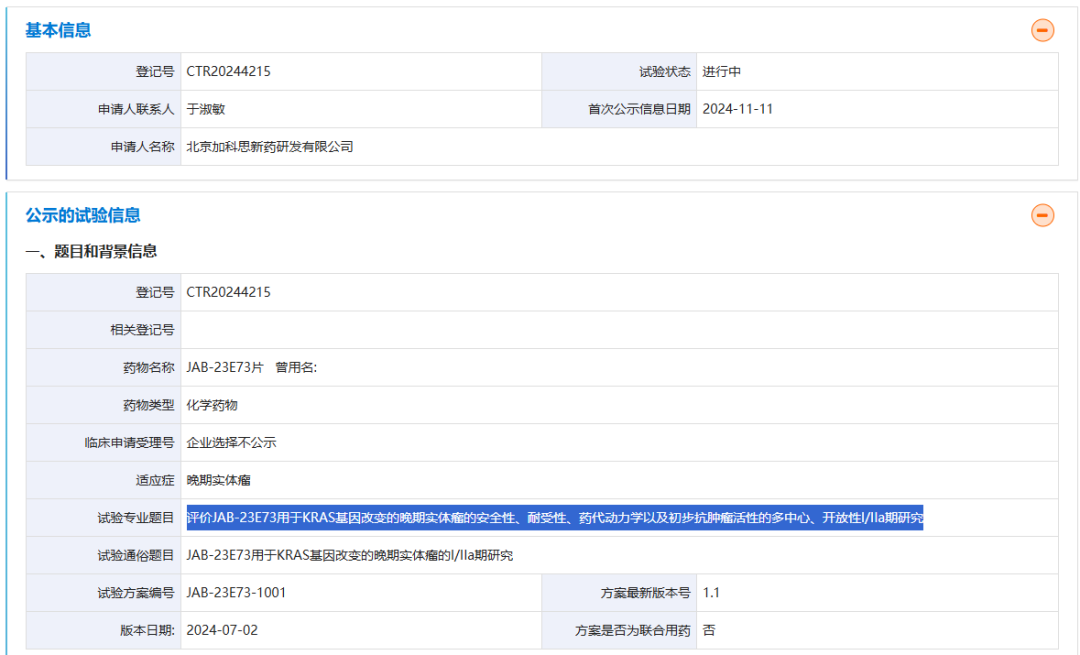
According to the Wise Fund APP, on November 11, the Drug Clinical Trial Registration and Information Disclosure Platform showed that Cisco (01167) registered a multicenter, open-label Phase I/IIa study to evaluate the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of JAB-23E73 for advanced solid tumors with KRAS gene mutations. This is the first clinical trial of the drug.
According to the Medicine Cube database, pan-KRAS inhibitors are currently in the early stages of clinical research. Globally, there are only 7 pan-KRAS inhibitors in Phase I clinical trials, including Pfizer's PF-07934040, BeiGene's BG-53038, and Eli Lilly and Co's LY4066434.
 In addition to JAB-23E73, Cisco has two other candidate drugs for KRAS inhibitors, namely the KRAS G12C inhibitor Golerese and the KRAS G12D inhibitor JAB-22000. Golerese has been applied for marketing and is under priority review, while JAB-22000 is in the preclinical research stage.
In addition to JAB-23E73, Cisco has two other candidate drugs for KRAS inhibitors, namely the KRAS G12C inhibitor Golerese and the KRAS G12D inhibitor JAB-22000. Golerese has been applied for marketing and is under priority review, while JAB-22000 is in the preclinical research stage.

 除了JAB-23E73外,加思科在KRAS抑制剂研发方面还有两款候选药物,分别是KRAS G12C抑制剂戈来雷塞和KRAS G12D 抑制剂JAB-22000,其中戈来雷赛已申报上市并被纳入优先评审,JAB-22000处于临床前研究阶段。
除了JAB-23E73外,加思科在KRAS抑制剂研发方面还有两款候选药物,分别是KRAS G12C抑制剂戈来雷塞和KRAS G12D 抑制剂JAB-22000,其中戈来雷赛已申报上市并被纳入优先评审,JAB-22000处于临床前研究阶段。