① In order to cope with market competition, Nanxin Pharmaceutical sold the core product paramivir at a reduced price, yet this move did not reverse the sharp decline in the company's revenue and net profit; ② Nanxin Pharmaceutical revealed that it has set up a major customer department to develop an out-of-hospital market specifically for products such as oseltamivir dry suspensions.
“Science and Technology Innovation Board Daily”, November 19 (Reporter Zheng Bingxun) “As market competition for paramivir sodium chloride injections gradually intensifies, the company gradually lowered the market price of this product. This move is conducive to consolidating market share.”
At the 2024 third quarter results briefing held on the 19th, Zhang Shixi, vice chairman and general manager of Nanxin Pharmaceutical (688189.SH), gave the above answers in response to investors' inquiries about price reductions for paramivir sodium chloride injections.
 However, from a more realistic perspective, Nanxin Pharmaceutical, which sells paramivir injections at reduced prices, has not performed well since this year.
However, from a more realistic perspective, Nanxin Pharmaceutical, which sells paramivir injections at reduced prices, has not performed well since this year.
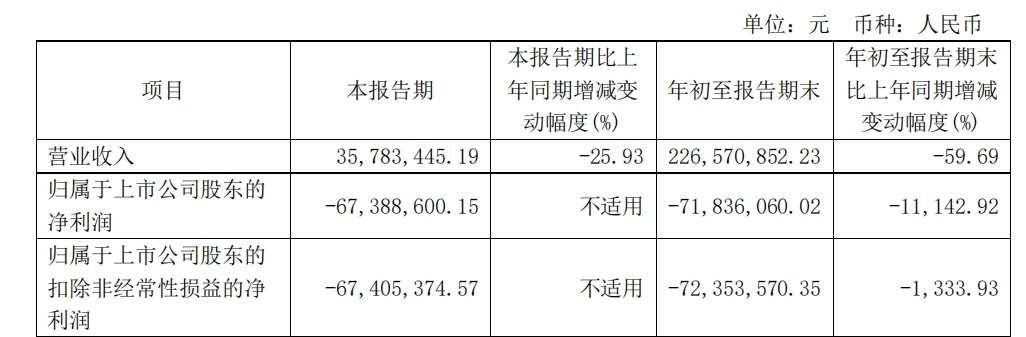
In the third quarter of 2024, Nanxin Pharmaceutical achieved revenue of 35.7834 million yuan, a year-on-year decrease of 25.93%, a net loss to mother of 67.3886 million yuan, and a net loss of 27.7578 million yuan for the same period last year. The first three quarters achieved revenue of 0.227 billion yuan, a year-on-year decrease of 59.69%, and a net loss to mother of 71.8361 million yuan, a sharp drop of 11142.92% over the same period last year.
Currently, Nanxin Pharmaceutical's revenue mainly comes from four categories of products, including anti-influenza virus drugs, cardiovascular drugs, antibiotics, and antipyretic and pain relievers. Among them, anti-influenza virus drugs are the core revenue source of Nanxin Pharmaceutical.
Furthermore, in 2020 and before, all sales revenue of Nanxin Pharmaceutical's anti-influenza virus drugs was contributed by paramivir injections. In 2020, revenue from peramivir reached 71.96% of total revenue. After 2020, Nanxin Pharmaceutical, which entered the Science and Technology Innovation Board, will no longer announce the specific sales situation of peramivir. However, sales of anti-influenza virus drugs are still the company's core revenue source.
In 2021-2023, sales revenue of anti-influenza virus drugs was 0.492 billion yuan, 0.462 billion yuan, and 0.667 billion yuan respectively, accounting for 66.09%, 66.05%, and 89.63% of Nanxin Pharmaceutical's overall revenue.
Nanxin Pharmaceutical once revealed to the outside world that the 5-year monitoring period for the innovative drug enjoyed by Paramivir injections expired in April 2018. Since Nanxin Pharmaceutical did not obtain a patent for the pharmaceutical compound, other pharmaceutical companies can synthesize paramivir by other methods, so there is a risk that this product will be copied domestically.
Currently, there are several competitive products of peramivir on the market. Furthermore, in this year's national health insurance negotiations, all 7 types of paramivir injections that passed the “Preliminary Examination Catalogue” entered the market on the last day of the negotiations.
In June 2023, Nanxin Pharmaceutical's other influenza product, oseltamivir phosphate suspension, was officially approved for marketing, changing the situation where it relied on paramivir to support revenue from anti-influenza virus drugs. At the time, Nanxin Pharmaceutical stated that it is expected to use existing sales channels to rapidly expand the market.
At the results meeting, the “Science and Technology Innovation Board Daily” reporter asked Zhang Shixi about the specific income situation of anti-influenza virus drugs in the first three quarters and how to deal with the current situation of various competing products participating in health insurance negotiations, but the other party did not respond to this.
However, in response to the expansion of the out-of-hospital market, Zhang Shixi revealed that Nanxin Pharmaceutical has set up a major customer department to develop the out-of-hospital market for products such as oseltamivir suspension, cefuroxime ester dispersible tablets, cefaclor suspensions, simvastatin dispersible tablets, ciprofloxacin sustained-release tablets, etc., and has now successfully solicited investment.
In addition, as early as 2022, Nanxin Pharmaceutical began arranging out-of-hospital sales for peramivir injections, and has now successfully invested in three terminal customers in 18 provinces and cities.
In addition to paramivir, Zhang Shixi also revealed that cefaclor capsules, selected in China, have continued to be successfully sold in 23 provinces and cities, and the exclusive compound hypotensive preparation benazepril hydrochlorothiazide tablets has been successfully promoted in 18 provinces and cities.

 然而从更现实的角度来看,采取对帕拉米韦注射液进行降价销售的南新制药,今年以来的业绩表现并不佳。
然而从更现实的角度来看,采取对帕拉米韦注射液进行降价销售的南新制药,今年以来的业绩表现并不佳。