① Microchip Biotech announced a fixed increase plan. It plans to raise 0.96 billion yuan, 0.71 billion yuan for innovative drug research and development, and 0.25 billion yuan to supplement working capital, with a backflow ratio of 26.04%; ② Multiple R&D pipelines allow Microchip Biotech to continue to burn money. The total R&D expenses for 2021-2023 are 0.927 billion yuan. Currently, there are 17 pipeline of indications in clinical trials.
“Science and Technology Innovation Board Daily”, November 21 (Reporter Zheng Bingxun) The original innovative pharmaceutical company Microchip Biotech (688321.SH) is about to finance again!
On the evening of the 20th, Microchip Biotech announced a fixed increase plan for 2024, which plans to raise 0.96 billion yuan. This is another time to raise capital after its 2019 IPO fundraising and refinancing the issuance of convertible bonds in 2021.
 According to the fixed increase plan, Microchip Biotech plans to use 0.71 billion yuan raised for “innovative drug research and development projects”, and the remaining 0.25 billion yuan will be used to supplement working capital, accounting for 26.04% of the backflow.
According to the fixed increase plan, Microchip Biotech plans to use 0.71 billion yuan raised for “innovative drug research and development projects”, and the remaining 0.25 billion yuan will be used to supplement working capital, accounting for 26.04% of the backflow.

Prior to that, of the 0.804 billion yuan raised by Microchip Biotech's IPO plan, 0.16 billion yuan was used to make up the flow, accounting for nearly 20%. At the time of issuing convertible bonds, out of the 0.5 billion yuan planned to be raised, 0.12 billion yuan was also used for reflow, accounting for 24% of the reflow.
Microchip Biotech does need to prepare more for cash flow. In 2019, Microchip Biotech listed on the Science and Technology Innovation Board, the balance of cash and cash equivalents on its account was $0.336 billion. In 2022, when the convertible bond issuance plan was registered and effective, the cash on the year-end account reached a new high of 0.415 billion yuan. However, by the end of 2023, Microchip Biotech's cash and cash equivalent balance was rapidly consumed to 0.267 billion yuan, and further consumed to 0.193 billion yuan by the end of September 2024.
The core reason for this situation is that Microchip Biotech is a biotech company, and it is also a biotech company whose main direction is to develop original innovative drugs. This determines that Microchip Biotech needs to continue to invest a high amount of money in research and development.
In 2021-2023, Microchip Biotech achieved revenue of 0.43 billion yuan, 0.53 billion yuan, and 0.524 billion yuan, respectively, and R&D investment of 0.234 billion yuan, 0.288 billion yuan, and 0.405 billion yuan respectively, accounting for 54.44%, 54.33% and 77.30% of revenue, respectively, and a total of 0.927 billion yuan in 3-year R&D expenses. In the first three quarters of 2024, Microchip Biotech achieved revenue of 0.481 billion yuan and R&D investment of 0.246 billion yuan, accounting for more than 51%.
According to data, Microchip Biotech has developed the world's first (first-in-class) and best-in-class (best-in-class) original novel drug. Two products have been approved for marketing with 5 indications. The products are sidabendamide and siglitazide, respectively.
In the Mainland, sidabendamide was approved for the three indications of recurrent or refractory peripheral T-cell lymphoma, advanced hormone-receptor-positive breast cancer, and diffuse large B-cell lymphoma in December 2014, November 2019, and April 2024, respectively.
Siglitazepam was approved later than sidabendamide. In October 2021 and July 2024, it was approved for the two indications of single-agent treatment of type 2 diabetes and the treatment of type 2 diabetes in combination with metformin.
Sidabenamide and siglitazide brought product sales revenue of 0.391 billion yuan, 0.483 billion yuan, and 0.509 billion yuan to Microchip Biotech in 2021-2023, accounting for 90.75%, 91.07%, and 97.16% of overall revenue.
In 2017, sidabenamide was included in the national medical insurance catalogue for the first time, and the unified retail price was lowered according to the standard of 385 yuan/tablet. In 2021, sidabendamide was renewed and entered the medical insurance catalogue, and the price was reduced to 343 yuan/tablet. In 2023, sidabendamide was renewed again, but the unit price was further reduced to 322.42 yuan/tablet.
However, although prices continued to decline, the gross margin of sidabenamide was not affected, which was 95.24%, 96.82%, and 96.09%, respectively, in 2021-2023.
In contrast, the profitability of siglitazepam is significantly inferior to sidabenamine. In January 2023, sitaglita sodium was included in the national medical insurance catalogue for the first time, and the retail price was lowered according to the payment standard of 2.92 yuan/tablet. The gross sales margin for that year was 15.44%.
On the 20th, Microchip Biotech also revealed the use of the previous fund-raising. Among them, it was revealed that the price reduction of siglitazide tablets was 68.15% when included in the medical insurance catalogue in 2023. This move also caused product revenue and profit to fall short of expectations.
Looking at it now, Microchip Biotech is clearly unable to support its huge operating expenses with the sales revenue of the two approved products. In order to enrich the product line, Microchip Biotech currently has at least 17 research and development pipelines for at least 17 indications, some of which have entered clinical phase III. At the same time, Microchip Biotech also has more than 10 pre-clinical small molecule products.
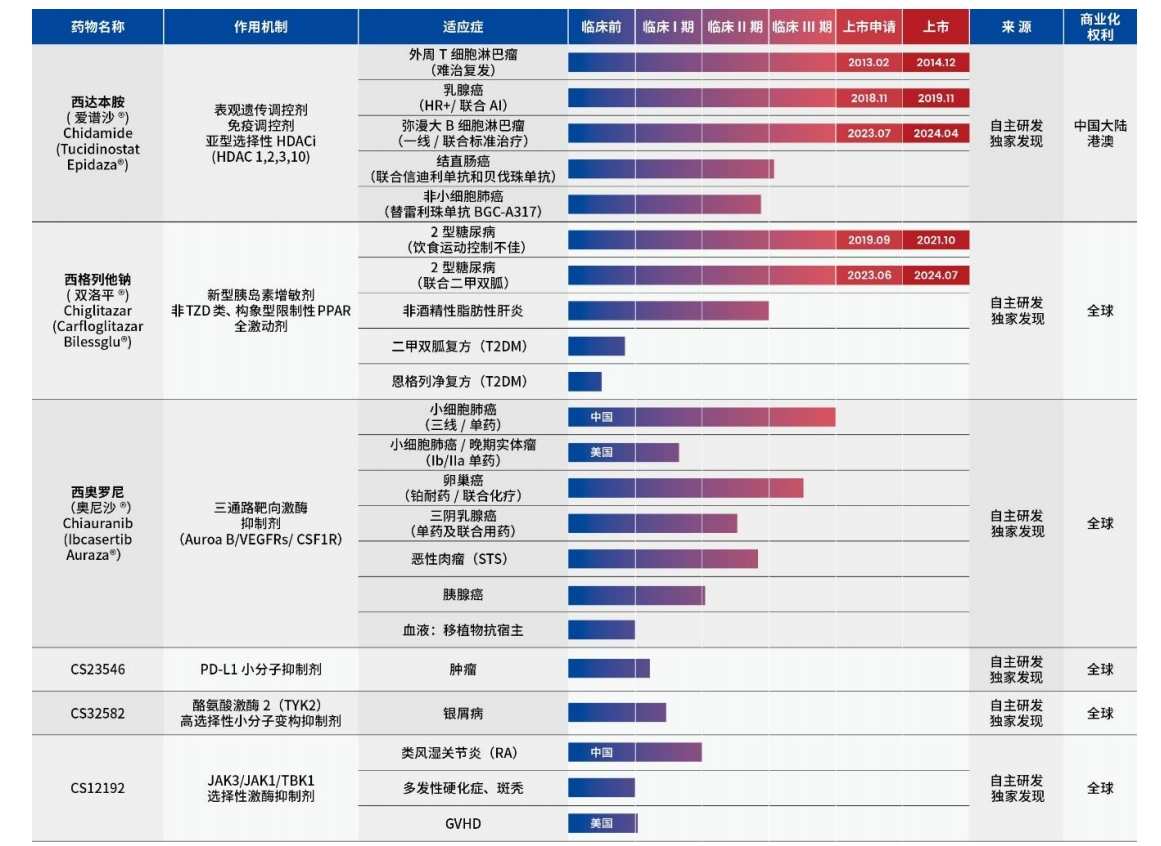
This time, the innovative drug research and development projects planned by Microchip Biotech will mainly focus on the current indications of sidabendamide and siglitazumab, including phase III clinical trials such as sidabendamide+cindirizumab plus bevacizumab in combination with colorectal cancer, sitaglitasone monotherapy for steatohepatitis (MASH) associated with metabolic dysfunction, and sidabendamide combined with PD-1 first-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) with positive PD-1 expression.

 根据定增预案,
根据定增预案,