GLP-1雙雄司美格魯肽和替爾泊肽,2024年合計銷售額將突破400億美元,由此催生的增肌藥物市場需求也是巨大的。
要站在巨人肩膀上。
GLP-1雙雄司美格魯肽和替爾泊肽,2024年合計銷售額將突破400億美元,由此催生的增肌藥物市場需求也是巨大的。
 禮來手握下一代藥王替爾泊肽,與之合作的CDMO、Biotech皆成贏家。禮來(LLY.US)正急切尋找替爾泊肽的伴侶藥物,ActRII抗體成爲最佳選擇之一。
禮來手握下一代藥王替爾泊肽,與之合作的CDMO、Biotech皆成贏家。禮來(LLY.US)正急切尋找替爾泊肽的伴侶藥物,ActRII抗體成爲最佳選擇之一。
一家中國Biotech站到禮來肩上。
禮來將負責在美國爲來凱醫藥ActRII增肌減脂藥物LAE102執行一項I期臨床研究並承擔相關費用,而來凱醫藥保留LAE102的全球權益。這裏面信息量好大,讓人一時緩不過來。
禮來爲何如此看重ActRII增肌減脂藥物?在重金收購全球進度第一的Bimagrumab(ActRIIA/B單抗)之後,又出錢出力爲全球進度第二的LAE102(ActRIIA單抗)做I期臨床,難道ActRIIA/B雙靶點還不如ActRIIA單靶點?
LAE102命運繫於I期臨床數據,如果符合預期,來凱醫藥(02105)將收穫多大金額的BD交易?
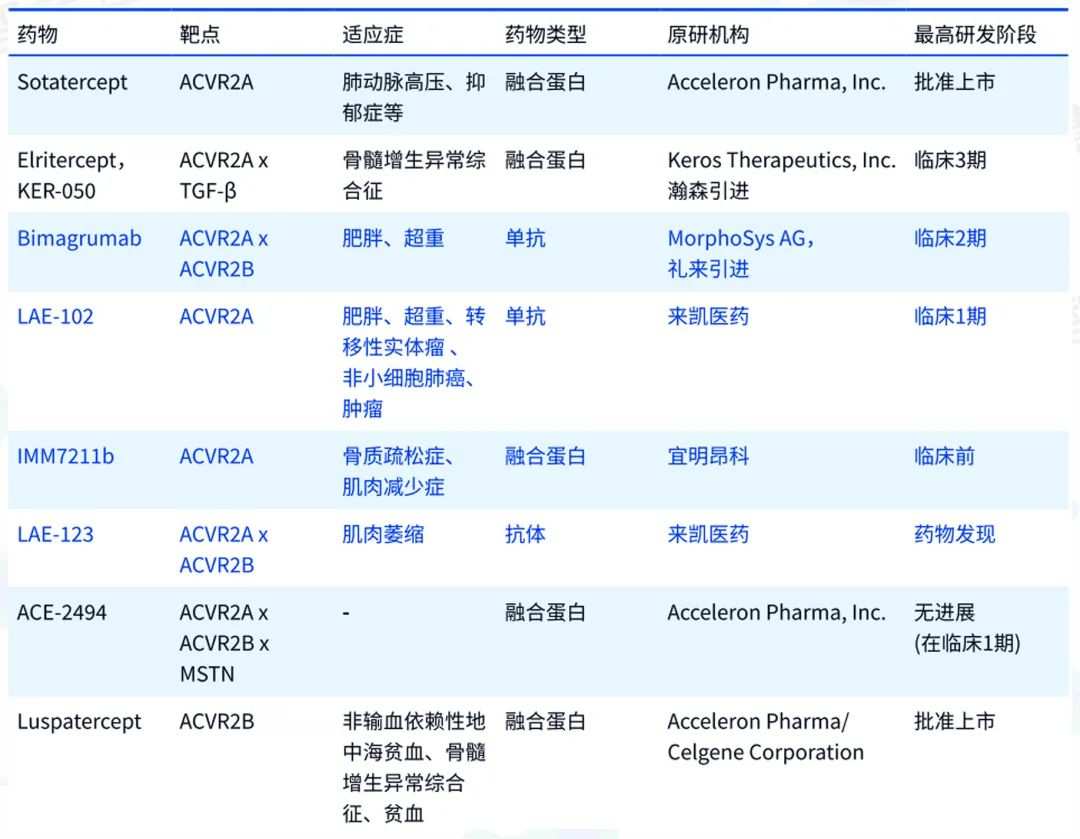
ActRII通路中的重點藥物 據智慧芽
01 超級身材的祕密
ActRII牛不牛,先看比利時藍牛。
比利時藍牛超級身材的祕密,源於生產肌肉生長抑制素(GDF-8)的基因發生突變失效,從而產生更多肌纖維。而ActRII是GDF-8的主要受體,抑制ActRII有望達到比單純抑制GDF-8類似或更佳的增肌效果。
ActRII存在於脂肪和肌肉細胞中,通過ActRII受體發出的信號會導致脂肪堆積和肌肉萎縮。脂肪組織中,通過 ActRII 受體的激活素信號直接促進脂質儲存,是內臟脂肪積累和肥胖的關鍵驅動因素,因而阻斷脂肪細胞中的ActRII信號傳導,可促進脂肪代謝;肌肉組織中,通過ActRII受體發出的信號會抑制肌肉生長並促進肌肉萎縮,因而阻斷骨骼肌中的激活素信號傳導可以促進肌肉再生。總之,阻斷ActRII 信號有望在減少脂肪堆積的同時,促進肌肉增加。
ActRII抗體是替爾泊肽的絕配,在增益減重效果同時,還能爲GLP-1RA藥物造成肌肉流失的缺點打上補丁。多項臨床研究和回顧研究的結果表明,GLP-1RA藥物受試者減掉的體重中近20-40%爲主要由肌肉質量構成的瘦體重(又稱去脂體重,Lean Mass),而肌肉流失會增肌患心血管疾病、骨質疏鬆症的風險。
2023年7月,禮來以19.25億美元收購Versanis Bio獲得ActRII增肌減脂藥物Bimagrumab。今年10月,禮來正式啓動Bimagrumab聯合替爾泊肽增肌減脂的II期臨床試驗。
在被禮來收購前,Bimagrumab的IIa期臨床試驗第48週數據顯示,可使受試者脂肪質量減少20.5%(減重效果與替爾泊肽基本相當),腰圍減少9.0厘米,瘦體重增加3.6%,而且患者在停止治療12周內並未觀察到體重反彈。
來凱醫藥創始人呂向陽博士是Bimagrumab的共同發明人。
02 藥王急尋伴侶
呂向陽對ActRII靶點研究已積累超過20年經驗,是全球權威專家。按照藥物迭代研發的特點,相比Bimagrumab,來凱醫藥LAE102在安全性和藥物機制方面應有改進,這可能也是吸引禮來兩邊下注的原因。
改進版
儘管靶向ActRII受體能夠全面阻斷骨骼肌生長抑制信號,但同時也會造成對多種配體的廣譜抑制,可能存在潛在的安全性隱患。
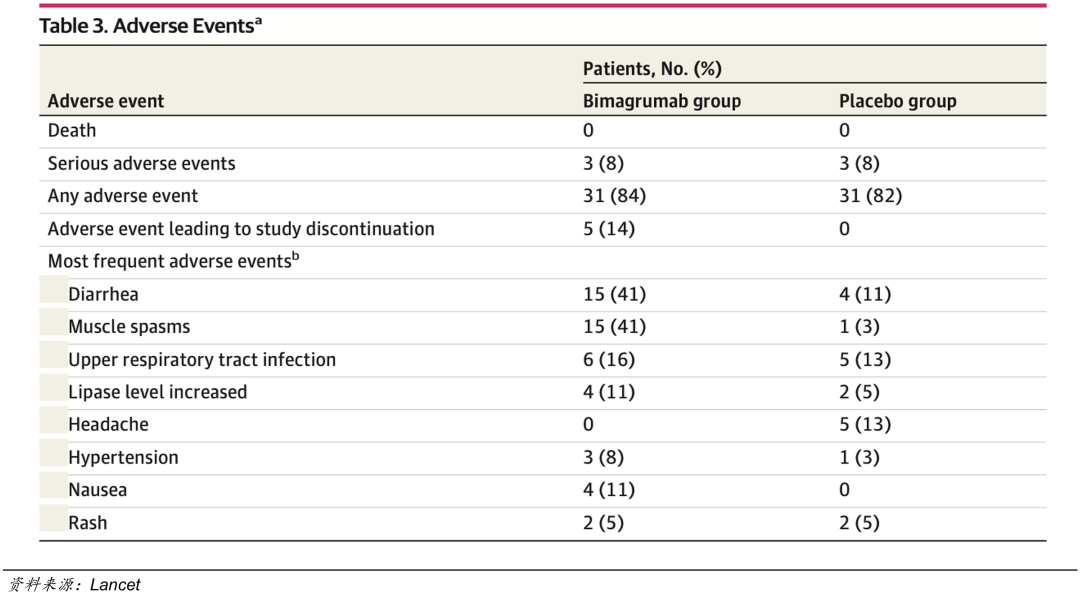
Bimagrumab臨床II期安全數據
Bimagrumab的副作用包括肌肉痙攣和腹瀉,發生在治療早期,在IIa期臨床試驗中,有5例患者(14%)因爲不良事件而導致研究終止。Bimagrumab是ActRIIA和ActRIIB雙靶點抑制劑,這些不良反應到底是哪個靶點引起的?相信禮來也非常希望進行更多驗證,所以有意通過臨床試驗進一步比較。
來凱醫藥基於ActRIIA/ActRIIB兩個Ⅱ型受體針對四個配體(ActivinA、ActivinB、GDF8、GDF11)的活性差異,以及整條通路在不同疾病中的作用,共佈局3款ActRII靶向藥物,LAE102(ActRIIA單抗)主攻增肌減脂,LAE103(ActRIIB單抗)、LAE123(ActRIIA/B單抗)適應症主要是肌肉萎縮症及其他疾病適應症。
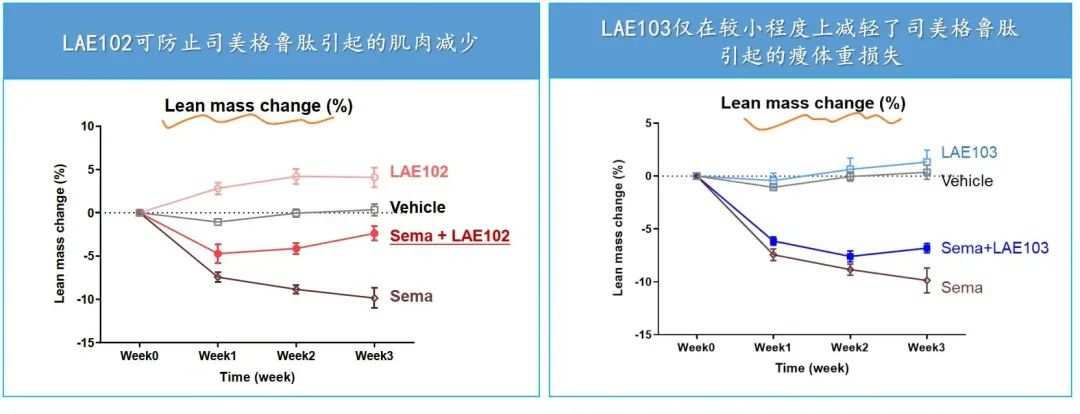
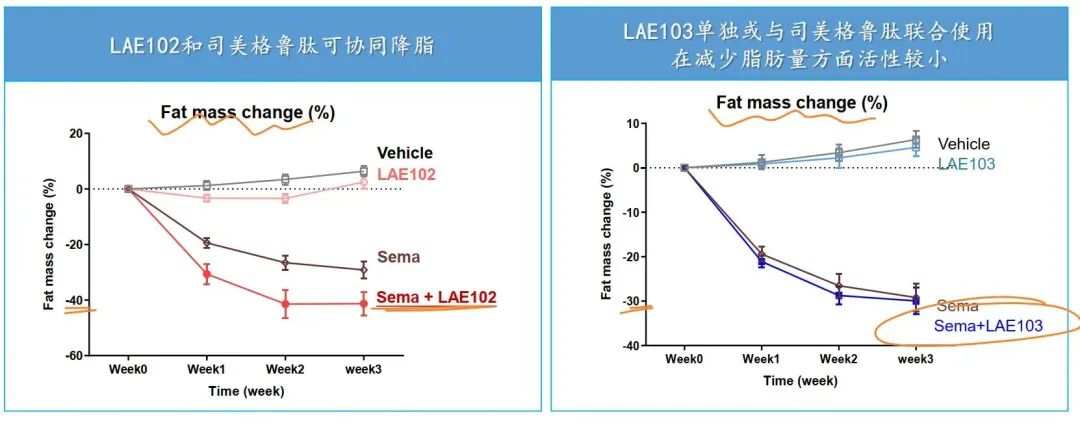
根據來凱醫藥公佈的DIO模型,LAE102和LAE103都對瘦體重有增加效果,而LAE102更顯著,並且LAE102與司美格魯肽可協同減脂。
LAE102選擇單靶向ActRIIA,這種相比Bimagrumab的差異化設計,有可能達到安全性更佳、同時增肌減脂的效果。
皮下注射
ActRII抗體作爲GLP-1RA伴侶藥物也應該可以皮下注射。
來凱醫藥則在今年10月啓動了LAE102單次劑量遞增研究的皮下注射(SC)部分,目標是2024年底前完成單次劑量遞增研究。
與靜脈注射給藥相比,皮下給藥對慢病患者長期使用更有優勢,也更易與GLP-GLP-1RA藥物聯用,對擁有GLP-1RA藥物的大藥企有更大吸引力。
BD金額
2024H1,全球25家Biotech被收購,其中6起併購案超過20億美元,15起併購案超過10億美元。收購Biotech的目標,當然是獲得對方的核心管線資產。
在減重創新療法領域,MNC出手更重,也越來越早。去年,Bimagrumab被禮來收購是在IIa期臨床完成、IIb期臨床入組的階段;今年10月,禮來14億美元佈局雙靶點減重療法,在KeyBioscience的DACRA(胰澱素/降鈣素受體雙重激動劑)新分子I期完成、II期還沒開始即出手。
BD的臨床階段越來越靠前,國內創新藥項目出海也在體現這一趨勢。據國盛證券統計,2023年中國創新藥License-out,臨床前項目佔比34%,I期臨床項目佔比26%,上市後項目佔比23%,II/III期臨床和申請上市階段佔比均低於10%。
據智慧芽數據,靶向ActRII通路的減重藥物全球進入臨床階段的僅有Bimagrumab和LAE102,稀缺資源引得禮來搶先佔位。
後GLP-1時代,龐大的增肌需求已不容置疑,ActRII抗體的增肌效果已由Bimagrumab驗證,懸念是安全性還有待後續更多臨床數據驗證。
LAE102的I期臨床數據讀出至關重要,期待一個超級BD。
來凱醫藥探索的是一種全新出海模式,MNC先點菜,再看數據買單,而Biotech高潛力管線資產在得到數據背書後,也有資格待價而沽。
GLP-1賽道越來越卷,來凱醫藥示範的是一種參與熱門賽道的正確方式,增肌減脂將是GLP-1賽道差異化和新一代產品競爭力的重點。
本文轉自微信公號「阿基米德Biotech」,智通財經編輯:李程

 礼来手握下一代药王替尔泊肽,与之合作的CDMO、Biotech皆成赢家。礼来(LLY.US)正急切寻找替尔泊肽的伴侣药物,ActRII抗体成为最佳选择之一。
礼来手握下一代药王替尔泊肽,与之合作的CDMO、Biotech皆成赢家。礼来(LLY.US)正急切寻找替尔泊肽的伴侣药物,ActRII抗体成为最佳选择之一。