China's National Medical Products Administration Pharmaceutical Evaluation Center (CDE) website has recently announced that hansoh pharma (03692)'s new third-generation EGFR-TKI drug, Gefitinib Mesylate Tablets, has had a new indication for market application accepted.
According to the Securities Times app, on November 27th, the China National Medical Products Administration Pharmaceutical Evaluation Center (CDE) website announced that hansoh pharma (03692)'s new third-generation EGFR-TKI drug, Gefitinib Mesylate Tablets, had a new indication for market application accepted. Public information shows that this is the fifth indication market application for Gefitinib Mesylate Tablets submitted in China.
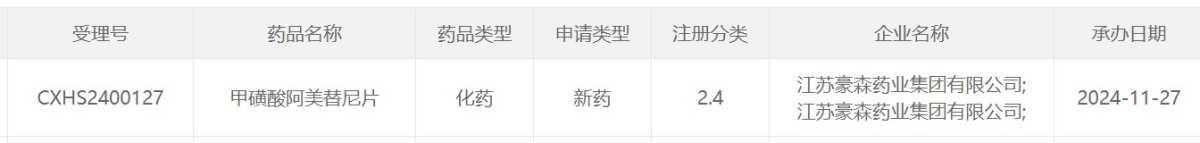
Screenshot source: CDE official website
 On October 22nd this year, hansoh pharma announced that a Phase III registration trial, AENEAS2, of Gefitinib Mesylate Tablets in combination with chemotherapy as a first-line treatment for patients with locally advanced (IIIB-IIIC stage) or metastatic (Stage IV) EGFR mutation non-small cell lung cancer (NSCLC) has achieved its primary endpoint of progression-free survival (PFS). Hansoh pharma stated that detailed data from this trial will be presented at future medical conferences and submitted to regulatory institutions. It is speculated that this may be the indication for market approval for Gefitinib Mesylate Tablets in this application.
On October 22nd this year, hansoh pharma announced that a Phase III registration trial, AENEAS2, of Gefitinib Mesylate Tablets in combination with chemotherapy as a first-line treatment for patients with locally advanced (IIIB-IIIC stage) or metastatic (Stage IV) EGFR mutation non-small cell lung cancer (NSCLC) has achieved its primary endpoint of progression-free survival (PFS). Hansoh pharma stated that detailed data from this trial will be presented at future medical conferences and submitted to regulatory institutions. It is speculated that this may be the indication for market approval for Gefitinib Mesylate Tablets in this application.

 今年10月22日,翰森制药宣布,一项阿美替尼片联合化疗作为局部晚期(IIIB-IIIC期)或转移性(IV期)EGFR突变非小细胞肺癌(NSCLC)患者的一线治疗方案的3期注册试验AENEAS2达到了其主要终点即无进展生存期(PFS)。翰森制药表示该试验的详细数据将在未来医学会议上发布及向监管机构递交。由此推测,这可能为本次阿美替尼申报上市的适应症。
今年10月22日,翰森制药宣布,一项阿美替尼片联合化疗作为局部晚期(IIIB-IIIC期)或转移性(IV期)EGFR突变非小细胞肺癌(NSCLC)患者的一线治疗方案的3期注册试验AENEAS2达到了其主要终点即无进展生存期(PFS)。翰森制药表示该试验的详细数据将在未来医学会议上发布及向监管机构递交。由此推测,这可能为本次阿美替尼申报上市的适应症。