Industry pessimistic expectations may improve.
Another significant bullish development in the biomedical sector, the A-shares in hk CRO concept has had a strong start this week.
As of the time of publication, A-shares of chempartner pharmatech, berry genomics, and others have hit the daily limit, while wuxi apptec has surged over 7%; wuxi bio in the Hong Kong stock market saw a 13% intraday increase, and wuxi apptec rose 12%, reaching the highest level in two months, though the gains have now retreated to 7%.
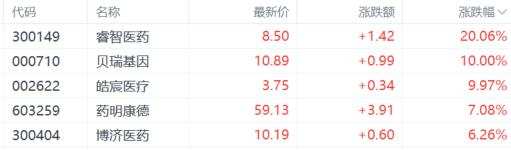
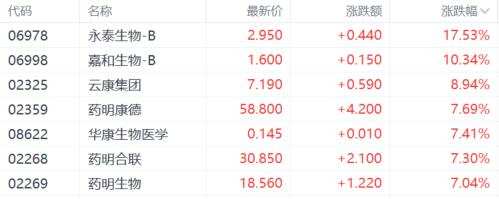
 On the news front, the final version of the NDAA from the USA House and Senate did not include the biosafety law 'Biosafety Act'.
On the news front, the final version of the NDAA from the USA House and Senate did not include the biosafety law 'Biosafety Act'.
This means that the five Chinese companies previously affected by the 'Biosafety Act' have gained an opportunity to breathe. However, according to process, this version must be signed by the President of the USA to take effect.
'Biosafety Act' legislation is off the cards for this year?
In fact, there were rumors last Friday that "us lawmakers are considering further measures on the biosafety bill," triggering a strong surge in biomedical.
Over the weekend, another bullish breakthrough occurred — the "Biosafety Bill" is very likely to be shelved in this year's legislation.
On December 7 local time, the military committees of both houses of the us Congress released the final agreement text of the National Defense Authorization Act (NDAA), and the "Biosafety Bill" was not included.
This year, both houses of Congress have been promoting the legislative procedures for the "Biosafety Bill" in their respective chambers, and there were also attempts to include the "Biosafety Bill" in the 2025 fiscal year NDAA.
Among them, five Chinese companies, including bgi genomics, wuxi apptec, wuxi bio, bgi smart manufacturing, and its subsidiary Complete Genomics, were specifically named.
In September, the House of Representatives voted overwhelmingly in favor of this "blacklist bill," and bipartisan groups in the Senate also expressed support.
Currently, there is no hope for the "Biosafety Bill" to be included in the NDAA, the congressional session is about to end, and it seems too late for the bill to be legislated separately this year.
Additionally, it is worth noting that the opposition to this bill within the us biomedical industry is steadily rising.
Some veteran Democratic members of the House of Representatives in the usa recently expressed their opposition to including the names of specific companies in the bill without due process.
A spokesperson for wuxi apptec also stated that they will continue to provide lawmakers and federal agencies with facts about the company and inform them of the valuable services it provides to clients in the usa and around the world.
Pessimistic expectations may improve.
Since the bill was proposed at the beginning of the year, the biomedical industry has also experienced several "shocks", with the concept represented by wuxi and other related sectors repeatedly riding the extreme market "roller coaster".
As the standalone legislation of the Biosecurity Act is stagnant, the industry's pessimistic expectations may improve.
Morgan Stanley stated that the National Defense Authorization Act (NDAA) for fiscal year 2025 in the usa does not include the Biosecurity Act or any names associated with wuxi, which is a positive development for the wuxi系 and other chinese CRO (Contract Research Organizations)/CDMO (Contract Development and Manufacturing Organizations) with significant overseas revenue exposure.
The latest report from lyons also pointed out that the usa Biosecurity Act was not included in the National Defense Authorization Act (NDAA), which greatly reduces the likelihood of the bill passing in 2024.
Considering the easing of geopolitical concerns and potential improvements in fundamental visibility, the brokerage believes this news is favorable for the cxO industry in china.
Similarly, Credit Lyonnais also stated that the likelihood of the usa reintroducing the Biosafety Act in 2025 is low. If this happens, a bill balancing drug research and development, manufacturing cost-effectiveness, and geopolitical factors is likely to be passed, rather than a complete decoupling.
However, CMB International stated that the usa Biosafety Act can still advance as separate legislation, but given that there are only 2 weeks left in the window, the success rate of separate legislation is extremely low.
In the long term, the institution is bullish on wuxi apptec maintaining a strong competitive advantage in the global medical research and development industry chain and sustaining a stable market share. In addition, it is expected that as global biomedical financing recovers, the company's orders will continue to grow rapidly, with the peptide business growing at high speed, driving performance recovery.
Additionally, there have been frequent recent national policies promoting openness in the medical field.
Industrial Securities pointed out that the focus should be on sub-sectors with good growth potential and industrial logic, with innovation + internationalization remaining the core keywords. In the medical sector, the technological attributes direction, innovative drugs remain the core theme, while also paying attention to parts of the innovative drug industry chain where the fundamentals are starting to improve and the subsequent risks are relatively small.
Additionally, medical instruments also conform to the logic of innovation + internationalization. Finally, if the economic fundamentals are expected to improve, cyclical varieties such as consumer medical will likely perform even more prominently.

