On December 23, Gelonghui reported that Zhejiang Wolwo Bio-Pharmaceutical (300357.SZ) announced that the company has recently obtained the "Drug Registration Certificate" approved and issued by the National Medical Products Administration. The registered products "Sycamore Pollen Allergens Skin Prick Solution", "German Cockroach Allergens Skin Prick Solution", and "Cat Dander Allergens Skin Prick Solution" have received approval for their market launch licenses.
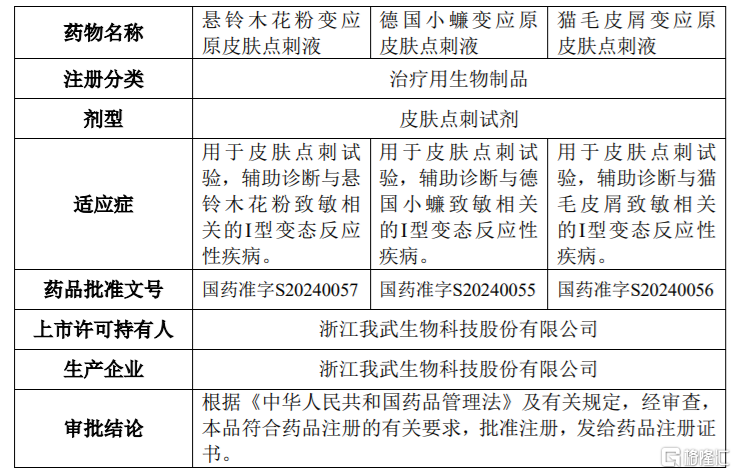
The three varieties of skin prick solutions, including Sycamore Pollen Allergens Skin Prick Solution, complement the company's existing products such as "Dust Mite Skin Prick Diagnostic Kit" (National Drug Standard S20080010), "House Dust Mite Skin Prick Diagnostic Kit" (National Drug Standard S20190022), "Artemisia Pollen Allergens Skin Prick Solution" (National Drug Standard S20230024), "Birch Pollen Allergens Skin Prick Solution" (National Drug Standard S20230023), and "Groundsel Pollen Allergens Skin Prick Solution" (National Drug Standard S20230025), which can meet the allergen testing needs of more allergy patients.
