Since reaching its peak in 2021, the overall performance of the Biomedical Sector has been less than optimistic. Even with the overall market being lively this year, the Biomedical Sector has still significantly underperformed the Index.
The performance of the capital markets reflects reality, where innovative pharmaceutical companies are facing issues such as a cooling in financing, a lack of financing channels, and tightening funds, which exacerbate the pressure on these companies to maintain continuous operations.
Recently, an exciting piece of news has arrived.
 The Hong Kong-listed company Ascentage Pharma (6855.HK) has obtained the overseas issuance and listing filing notice from the China Securities Regulatory Commission, with plans to issue no more than 33,739,347 shares of common stock and list on the Nasdaq in the USA.
The Hong Kong-listed company Ascentage Pharma (6855.HK) has obtained the overseas issuance and listing filing notice from the China Securities Regulatory Commission, with plans to issue no more than 33,739,347 shares of common stock and list on the Nasdaq in the USA.
At the same time, the company announced on the Hong Kong Stock Exchange on December 29 that it had publicly submitted its F-1 registration statement to the U.S. Securities and Exchange Commission regarding the proposed initial public offering of American Depositary Shares representing its common shares. The company plans to apply for a listing on the Nasdaq Global Select Market under the stock code "AAPG." J.P. Morgan Securities LLC and Citigroup Global Markets Inc. will serve as the joint bookrunners and underwriters for this issuance. The announcement also reconfirmed the aforementioned filing matters.
Thus, Ascentage Pharma has become the first biomedical company approved by the China Securities Regulatory Commission to list in the USA since the beginning of 2024. It is also the first Hong Kong 18A listed company to publicly submit an application for listing in the USA.
Since the announcement of the IPO filing news, it can be seen that the stock price of Ascentage Pharma has also shown a strengthening trend recently, indicating that the market is bullish on this news.
It is worth mentioning that this year, Ascentage Pharma's share price increased by more than 200% from its low to high within the year. As of now, the company ranks fourth in the growth of HEALTH CARE B among Hong Kong stocks, indicating the market's favor towards it.
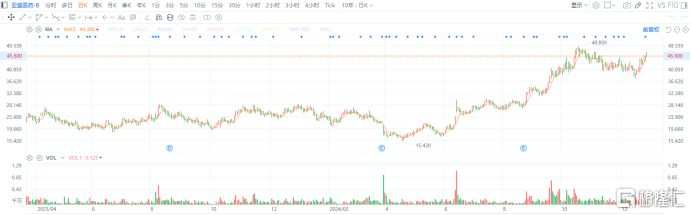
(Source: Futu Market)
So, with Ascentage Pharma's IPO registration approved by the domestic Securities Regulatory Commission, its listing in the USA is expected to accelerate. How should the opportunities behind this be viewed?
1. The fourth innovative drug company listed in both Hong Kong and the USA enters a new milestone.
As mentioned above, in recent years, under the capital winter in the Biomedical Industry, innovative drug companies have faced ongoing pressure in financing.
Relevant data shows that the financing amounts in the domestic medical and health sector from 2020 to 2023 were 245.9 billion yuan, 219.2 billion yuan, 125.8 billion yuan, and 82.9 billion yuan respectively. It is expected that in 2024, the total scale may fall below 10 billion USD.
For the Biomedical Industry, which is characterized by high investment, high risk, and high output, the demand for funds has always been a key factor in its development.
Despite the fact that from the perspective of New Journey Health Technology Group, the company has products that have already been commercialized to supplement its cash flow, and in July this year, it reached a major cooperation with the multinational pharmaceutical giant Takeda Pharmaceutical, receiving nearly 1.3 billion in cash, the overall financial pressure is not significant. However, from a long-term perspective, this sprint to list on the Nasdaq for financing is of great significance for its long-term development.
On the one hand, from the perspective of the Hong Kong stock market, in recent years, affected by factors such as the low valuations given to pharmaceutical companies and poor liquidity in this market, many pharmaceutical companies have faced obstacles in financing, leading to a wave of privatization and delisting.
It can be said that New Journey Health Technology Group is proactively preparing by choosing to venture to the USA.Secondary listing.This not only allows it to respond in advance to the challenges of the HK stock market, but also considering that the valuation given to innovative pharmaceutical companies in the Nasdaq market is generally higher, it will also help the company to achieve a revaluation, further gaining market recognition and financial support.
In fact, there are currently only 3 biomedical companies listed both on Nasdaq and HK, and their market caps are all significant. This also means that such companies carry a certain "scarcity" label in the capital markets, which helps them to gain more attention and support from the market.
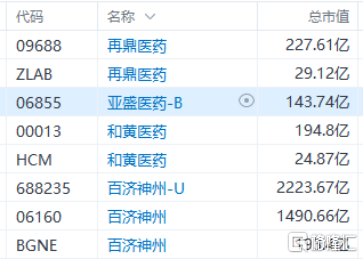
(Source: Futu Market)
On the other hand, going public in the USA can further open the door to the global capital markets. Utilizing the advantages of the US capital market helps to expand financing channels while enhancing brand visibility, promoting international cooperation, exploring overseas markets, integrating into the global Biotechnology Industry Chain, and improving global competitiveness.
In this regard, a research report by Founder Securities at the end of November previously pointed out that Asymchem plans to go public in the USA, which is expected to open a new development phase, helping to expand financing channels and integrate into the global Biotechnology Industry Chain. At the same time, it can not only further enrich cash on hand but also hopes for a valuation reassessment in the US stock market. Founder Securities' initial coverage also gave the company a "strong buy" rating and estimated the current reasonable valuation of the company to be 22.9 billion yuan based on the DCF valuation model. It is evident that professional investment institutions are bullish on it.
2·The "Global New" pipeline has strong potential and is entering a window for value release.
In the current capital winter of the Biomedical industry, the capital markets are increasingly focused on identifying directions with certain opportunities. Although the Innovative Drugs industry has a high-risk attribute of "great achievements coming at the cost of many losses," the understanding of innovation has begun to shift from purely the pursuit of technology to a focus on efficiency and certainty.
Against this backdrop, innovative drug companies with confirmed commercial success and international realization expectations are clearly more likely to gain the favor of capital.
Focusing on Asymchem, the company’s successful commercialization not only provides stable cash flow but also offers the capital markets a certain return expectation to some extent.
As one of the few 18A companies in the Hong Kong stock Biomedical sector that has commercialization capabilities, its first listed product, NAI Li Ke, shows high value potential. Currently, all approved indications for NAI Li Ke have been included in the national medical insurance catalog, and it is expected to maintain strong growth. In the first half of 2024, NAI Li Ke achieved sales revenue of 0.113 billion yuan, a 120% increase compared to the second half of last year.
From the specific data of the Earnings Reports, 2024 is a turning point year for Asymchem, thanks to progress in business development and commercialization. The company started to achieve profitability for the first time in the first half of this year, realizing 0.824 billion yuan in revenue and 163 million yuan in profit in the first half of the year. The company’s financial position is stable, with cash on hand reaching 1.8 billion yuan. It can be said that both the safety and stability of operations are adequately guaranteed.
It is worth mentioning that the overseas licensing of Nilotinib has brought greater certainty to its short-term and long-term performance growth. According to the collaboration between Ascentage Pharma and Takeda Pharmaceutical, as Nilotinib progresses globally in clinical trials and is launched overseas, Ascentage Pharma is expected to receive more funding in the coming years. In this regard, the report from Founder Securities mentions that the overseas Nilotinib treatment for CML is expected to complete the enrollment of the Phase III clinical trial POLARIS-2 for US registration by 2025, with a new drug application expected to be submitted in the US in 2026, and it could be approved for market launch as early as 2026-2027. Thereafter, Takeda's mature hematological oncology sales team will drive the commercialization overseas, with the $1.2 billion option exercise fee + additional milestone payments, as well as a gradually realized double-digit percentage revenue sharing from annual sales.
From this perspective, the company's subsequent profits are expected to become normalized, and the figures will continue to amplify. For the capital markets, the added certainty undoubtedly helps to obtain more market premium opportunities, which is also an important logic behind the company's ability to 'stand out from the crowd' in the capital market this year.
(Source: Futu Market)
From a long-term perspective, the verified pipeline R&D capabilities and commercialization abilities, as well as holding multiple heavyweight 'global new' products in development, especially the established foundation in the hematological oncology field, determine that the company will continue to have plenty of highlights in the future.
Ascentage Pharma has a self-developed protein-protein interaction targeted drug design platform, placing it at the forefront globally in the development of new drugs in the apoptosis pathway. The varieties in the company's pipeline also possess 'first-in-class' and 'best-in-class' potential, targeting the global market.
Firstly, continue to pay attention to its core product Nilotinib.
As the first third-generation BCR-ABL inhibitor approved for market launch in China, Nilotinib is a Best-in-class innovative drug globally, with outstanding competitiveness in the hematological oncology field, and has been selected for an oral presentation at the American Society of Hematology (ASH) annual conference for the seventh consecutive year, showcasing the international hematology community's recognition of its efficacy and safety.
As early as November 2021, Nairlic's first indication was approved, making it the first treatment drug in China for chronic myeloid leukemia (CML) resistant to TKI and accompanied by a T315I mutation, breaking the clinical treatment void. In November 2023, Nairlic's new indication was approved again for the treatment of CML-CP patients who are resistant and/or intolerant to first- and second-generation TKIs, further expanding its treatment scope.
It is noteworthy that in November of this year, Nairlic's new indication was included in the national medical insurance through a simplified renewal process, meaning that all currently marketed indications of this drug have been included in the national medical insurance drug catalog. With increasing market demand and supportive policies, there is a continuous expansion in capacity, realizing performance growth. This also fully demonstrates the clinical value of this drug and its recognition by the terminal market. In this regard, a viewpoint from Founder Securities pointed out that compared to the first indication for CML patients with T315I mutation resistance, the number of patients covered by the new indication is expected to expand nearly 3-5 times, making sales volume anticipated for 2025. Additionally, at the recently concluded 2024 ASH annual meeting, impressive data on Nairlic for the second-line treatment of non-T315I mutation CML-CP patients was presented for the first time, indicating that Nairlic is expected to advance toward frontline treatment.
In addition, Nairlic has shown significant efficacy in overseas studies for patients with recurrently treated CML, especially for those who are resistant/intolerant to Ponatinib or Asciminib, and it is expected to fill the unmet needs in global CML treatment. The drug has currently received four orphan drug designations (ODD) from the FDA for chronic myeloid leukemia (CML), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), and gastrointestinal stromal tumors (GIST), as well as a fast track designation (FTD), and has also obtained an orphan drug designation from the EMA (European Medicines Agency) for the treatment of chronic myeloid leukemia. Its overseas clinical progress has been published multiple times in international authoritative journals and academic conferences, showcasing the pharmaceutical industry's high recognition of this globally promising Best-in-class potential drug. In February 2024, Nairlic received FDA approval to initiate a global registration Phase III clinical trial for the treatment of treated CML patients, marking another success for its international clinical development.
Ascentage Pharma is also actively expanding its global layout, continuously increasing the influence of Nairlic, and seeking opportunities for global development. The collaboration with Takeda Pharmaceutical is bringing substantial imaginative space for the company. Through this collaboration, not only does it provide financial support to Ascentage Pharma, but it could also leverage Takeda's global network and resources to accelerate the global clinical development and commercialization process of its products.
On the other hand, several other heavyweight pipelines with global competitiveness are also gaining momentum.
For example, the second product APG-2575, which is about to be commercialized and most anticipated by the market, is the first domestically developed Bcl-2 inhibitor submitted for NDA in China and is expected to become the second approved Bcl-2 inhibitor globally. This drug is the next core product with billion-dollar molecule potential for Ascentage Pharma after Olverembatinib. In addition to the impending commercialization in the chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) field, APG-2575 exhibits robust potential in the therapeutic areas of acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and multiple myeloma (MM), and is currently undergoing multiple global registration Phase III clinical trials.
It is reported that developing drugs targeting Bcl-2 is extremely difficult; nearly 40 years have passed since the target was discovered, yet only one Bcl-2 inhibitor, AbbVie's Venetoclax, was approved in 2016. From the market landscape perspective, Venetoclax remains the only approved Bcl-2 inhibitor globally, with sales increasing from $0.018 billion at the start of its launch in 2016 to $2.288 billion in 2023, and reaching $1.251 billion in the first half of this year, currently still in a phase of rapid growth.
Considering the potential heavyweight bomb that Ascentage Pharma holds, as the fastest to market domestic Bcl-2 inhibitor, APG-2575 is expected to break Venetoclax's market monopoly and usher in a new leap in performance. Consequently, a research report from Founder Securities pointed out that APG-2575 for the treatment of R/R CLL/SLL is expected to be launched in 2025, with 1.5 line treatment for CLL anticipated for the U.S. market as early as 2027-2028; first-line treatments for AML and MDS are projected to be launched domestically in 2028 and for MDS in the U.S. market in 2028-2029, with total peak sales reaching 9.309 billion yuan. It is evident that the subsequent uplift on the company's performance will be substantial.
It is also noteworthy that Bcl-2 inhibitors have broad application prospects in hematological tumors, and their market launch has once changed the treatment landscape for various blood cancers. Currently, Ascentage Pharma's APG-2575 has been explored in multiple hematological cancer areas including chronic lymphocytic leukemia (CLL), AML, and WM, and it is expected to become a cornerstone drug for the next generation of hematological tumors, bringing more new treatment options to the field.
Finally, Ascentage Pharma's research pipeline also includes Other products with First-in-Class or Best-in-Class potential, such as MDM2-p53 inhibitor APG-115 and EED inhibitor APG-5918, and the development of these products is actively being advanced, further enriching the company's portfolio of Innovative Drugs and opening up future growth potential.
It is worth mentioning that at this year's ASH annual meeting, in addition to Nilotinib, APG-2575, APG-2449, and APG-5918 also made it to the presentation and report list, fully demonstrating Ascentage Pharma's influence in the global hematology community.
Ascentage Pharma's global layout and R&D capabilities are continuously strengthening. As of June 30, 2024, the company holds 520 authorized patents worldwide, of which 367 are overseas patents. Ascentage Pharma's product pipeline has conducted over 40 clinical trials in China, the USA, Australia, Europe, and Canada, continuously gaining global recognition.
3·Timing, geographical advantage, and favorable conditions await a new leap in value.
Looking at the present, going public in the USA is indeed the right time and place; Ascentage Pharma is expected to usher in a new round of value leap.
On one hand, from a policy perspective, the country actively promotes the development of Innovative Drugs, providing a favorable external environment for innovative pharmaceutical companies like Ascentage Pharma.
As early as July 5 of this year, the State Council reviewed and approved the "Implementation Plan for Supporting the Development of Innovative Drugs throughout the entire supply chain," and subsequently, various provinces and cities followed up with relevant policies. These policies include not only direct financial support but also cover various aspects such as price management, medical insurance payment, commercial insurance, drug distribution and usage, and investment financing, forming a strong support for the full supply chain of Innovative Drug R&D, production, and sales. In addition, multiple levels of pilot programs at the national and provincial levels are also continuously introducing measures to accelerate the review and approval of innovative drugs and to establish support funds related to Biomedical.
It is evident that a series of bullish policies have undoubtedly provided policy support and positive market expectations for innovative pharmaceutical companies like Ascentage Pharma, which helps enhance their attractiveness in the capital markets.
On the other hand, the long-silent biomedical sector is expected to迎来新的拐点 under the bullish expectation of a bull market in Chinese assets.
As is well known, the overall valuation of the pharmaceutical sector has continued to be under pressure over the past few years, both in terms of funding allocations and valuations, which remain at low levels. The industry sentiment has not fully recovered. Now, with the current Federal Reserve's interest rate cut cycle and the introduction of more domestic policies to stabilize growth, China's assets, as a valuation undervaluation, are expected to attract more international capital inflow and experience value reassessment.
In this context, the value of the biomedical sector will become more elastic and attractive due to policy support, performance rebound, and events driven by overseas expansion. For Ascentage Pharma, the advantages of being listed in the US and Hong Kong will enable it to face international investors directly, while the connectivity advantages of the Hong Kong and US markets will also allow it to capture more market opportunities.
Returning to Ascentage Pharma itself, the company holds a significant pipeline, continuously fulfilling operational expectations, and has many attractive value catalysts. It possesses high market recognition and scarcity; both from the safety brought by cash flow and the long-term potential driven by pipeline commercialization, demonstrating strong attractiveness.
In fact, from a long-term perspective, Ascentage Pharma's global innovation capability aligns with the growth logic of the US biomedical sector, which also determines that its long-term value still has considerable room for imagination.
It can be seen that Ascentage Pharma's R&D focus includes small molecule inhibitors of key apoptotic pathways such as Bcl-2, IAP, and MDM2-p53, which play an important role in cancer treatment and possess significant commercial value potential. The company has established a unique high-barrier technology platform through its global development strategy, and this underlying advantage provides the company with long-term growth potential. Moreover, Ascentage Pharma's global clinical trial layout not only accelerates the R&D process of new drugs but also lays a solid foundation for its global collaboration and market expansion, enabling the company to continuously realize its value growth potential.
Notably, at the beginning of this month, Ping An Securities also released research reports showing a bullish outlook for the company. It mentioned that the company's core product, Olverembatinib, has been included in the medical insurance system domestically, continuously expanding patient coverage, and a strategic collaboration with Takeda Pharmaceutical for the global layout of its products has been established. APG-2575 is expected to become the second BCL-2 inhibitor listed globally, with multiple pivotal phase 3 studies progressing smoothly. The company has submitted its listing application to the SEC in the USA, advancing towards the international stage. It is estimated that from 2024 to 2026, the company will achieve revenues of 0.96/0.41/2.93 billion yuan. Considering that the company has not yet entered the profitability stage, a DCF method is used to value the company, corresponding to a Market Cap of 20.7 billion HKD, and the initial coverage gives a 'recommendation' rating.
4. Conclusion
The year 2024 can be seen as a year in which the domestic innovative drug ecosystem enters a new stage, with a series of policy changes indicating that supporting innovation has become a consensus for the future high-quality development of the entire HEALTH CARE Industry.
As a benchmark enterprise in the Industry that has always regarded innovation as a standard, Ascentage Pharma's uniqueness lies in its "technological obsession" while also emphasizing efficiency and stable Operation, which clearly has a stronger adaptability to capital in the current context where capital seeks certainty.
From the beginning, the company has positioned itself on a "Global Innovation" route centered on patients, continuously building high competitive barriers, while its strong execution capability and resource synergy allow the company to quickly realize the commercial outcomes of heavyweight products and achieve a self-sustaining closed loop. In the subsequent development, the company has a series of blockbuster products accelerating their advancement, expected to provide ample momentum for the company's performance.
As the company's global layout gradually takes shape, its performance growth space is also continuously opening up. With the dual drive of favorable policies and market demand, Ascentage Pharma is expected to achieve a leap in value in the new stage of domestic innovative drug development and become an important participant in the global innovative drug field.

 港股上市公司亚盛医药(6855.HK)已获得中国证监会境外发行上市备案通知书,公司拟发行不超过33,739,347股普通股并在美国纳斯达克证券交易所上市。
港股上市公司亚盛医药(6855.HK)已获得中国证监会境外发行上市备案通知书,公司拟发行不超过33,739,347股普通股并在美国纳斯达克证券交易所上市。