AstraZeneca (AZN.US) has launched Phase III clinical trials for its PD-1/TIGIT dual antibody.
According to Zhitong Finance APP, on January 3rd, the official platform for drug clinical trial registration and information disclosure indicated that AstraZeneca (AZN.US) has registered a global Phase III study (CTR20244980) for Rilvegostomig or the combination of Pembrolizumab and chemotherapy as first-line treatment for metastatic non-squamous non-small cell lung cancer.
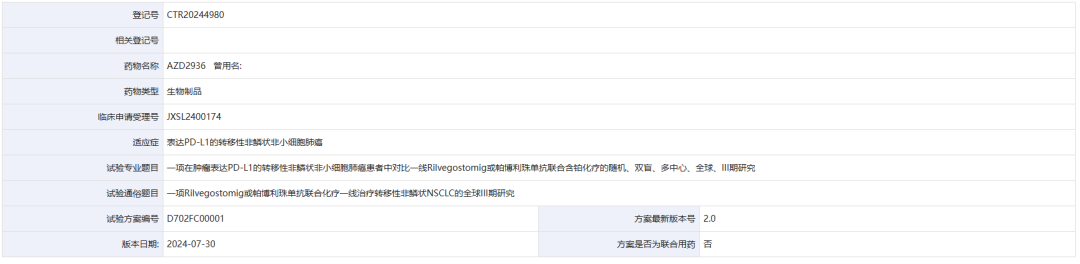
Screenshot source: Drug clinical trial registration and information disclosure platform.
 This is a randomized, double-blind, multicenter, global Phase III study designed to evaluate the efficacy and safety of Rilvegostomig combined with chemotherapy compared to Pembrolizumab combined with chemotherapy in first-line treatment for tumor expressing PD-L1 (TC≥1%) in non-squamous non-small cell lung cancer participants. The primary endpoints are OS and PFS. A total of 250 institutions are participating in this trial, with a plan to enroll 132 subjects domestically and 878 subjects internationally.
This is a randomized, double-blind, multicenter, global Phase III study designed to evaluate the efficacy and safety of Rilvegostomig combined with chemotherapy compared to Pembrolizumab combined with chemotherapy in first-line treatment for tumor expressing PD-L1 (TC≥1%) in non-squamous non-small cell lung cancer participants. The primary endpoints are OS and PFS. A total of 250 institutions are participating in this trial, with a plan to enroll 132 subjects domestically and 878 subjects internationally.

 这是一项随机、双盲、多中心、全球、III 期研究,旨在评估 Rilvegostomig 联合化疗与帕博利珠单抗联合化疗相比一线治疗肿瘤表达 PD-L1(TC≥1%)的非鳞状非小细胞肺癌参与者的有效性和安全性。主要终点是 OS 和 PFS。该试验共有 250 家机构参与,国内计划入组132名受试者,国际878名受试者。
这是一项随机、双盲、多中心、全球、III 期研究,旨在评估 Rilvegostomig 联合化疗与帕博利珠单抗联合化疗相比一线治疗肿瘤表达 PD-L1(TC≥1%)的非鳞状非小细胞肺癌参与者的有效性和安全性。主要终点是 OS 和 PFS。该试验共有 250 家机构参与,国内计划入组132名受试者,国际878名受试者。