Mechanical thrombectomy has become a favorable weapon in addition to conventional intravenous thrombolysis in the treatment of ischemic stroke.
36Kr Holdings learned thatWemu Biological Engineering Co., Ltd.(hereinafter referred to as "We Mu Bio") has completed tens of millions of US dollars in round B financing, led by Hillhouse Venture Capital, followed by Junlian Capital and Sanzheng Health Investment, and Haoyue Capital served as the exclusive financial adviser for this round of financing.This financing will be used to support the development and introduction of follow-up new products.Rapid researchThe products in the distribution pipeline are promoted to the commercialization of clinical and listed products.
Wo Mu Bio is a platform company specializing in innovative solutions in the field of vascular intervention, with a current product line covering the treatment of ischemic stroke, hemorrhagic stroke and intracranial vascular stenosis.
 It is reported that the company's listed product TracLine is a new generation of distal access catheter, which is suitable for all kinds of nerve interventional surgery; in addition, its core product, intracranial thrombus aspiration catheter, is about to enter the market.In the future, pathway products based on complete stroke solutions and related therapeutic products with cutting-edge technology will be launched one after another.
It is reported that the company's listed product TracLine is a new generation of distal access catheter, which is suitable for all kinds of nerve interventional surgery; in addition, its core product, intracranial thrombus aspiration catheter, is about to enter the market.In the future, pathway products based on complete stroke solutions and related therapeutic products with cutting-edge technology will be launched one after another.
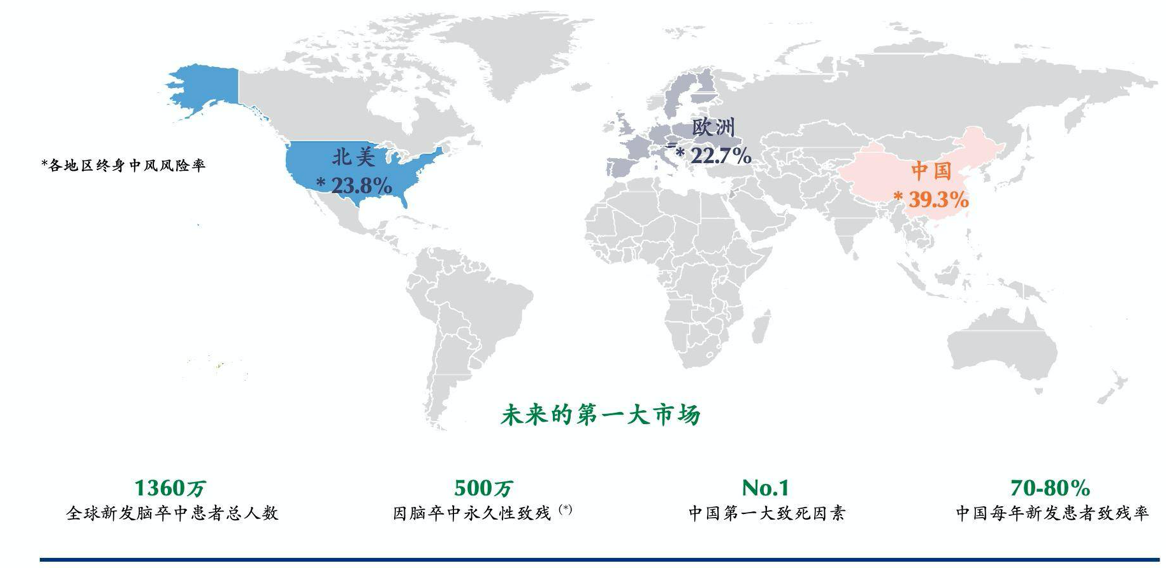
Stroke is the second largest fatal disease in the world and the first in China, with high mortality, high morbidity, high recurrence rate and high disability rate. If the patient can not get effective treatment, it will cause serious psychological and economic burden to the family and society.
China will become the largest market for neural intervention in the world.
Haoyue Capital supply Map
The field of neural intervention in China started later than that in Europe and the United States and other developed countries, but with the continuous development of imaging technology and vascular intervention technology, neural interventional devices have developed rapidly in the Chinese market in the past few years. and due to the aging of society, the improvement of the overall level of diagnosis and patient awareness and the accelerated construction of national stroke centers, this trend will continue.
According to statistics, the market size of neural interventional devices in China is 4 billion yuan in 2018, and it is expected that by 2025, the figure will exceed 20 billion yuan, with a compound growth rate of 26%. At the same time, China is also likely to surpass other developed countries in the world to become the world's largest single market in the field of nerve intervention in the field of high-value consumables.
In the treatment of ischemic stroke, it has been emphasized internationally that "time is the brain". If the patient can arrive at the hospital one minute earlier, the follow-up effect will be very different. In recent years, many cities have released stroke first aid maps to create an one-hour "golden circle" to directly locate the nearest stroke center.
With the publication of the "five RCT" trials in 2015, mechanical thrombectomy has become a useful weapon in addition to conventional intravenous thrombolysis in the treatment of ischemic stroke. The Compass trial published by Professor Turk in the Lancet in 2019 well verified the effectiveness and economic value of ADAPT (a direct aspiration first-pass thrombectomy, direct contact for the first time) technology for thrombectomy.
At present, more than half of the endovascular treatment of ischemic stroke in the United States market has adopted ADAPT technology, which is consistent with the layout of the core products of cereal biology.
Dr. Wang Jicheng, founder of Wo Mu Biology, said that at present, the existing technologies and products in the domestic neural intervention market can not fully meet the needs of clinicians and patients. with their own experience and resources in the industry, the company has chosen to overcome the most challenging catheter research and development technology and carry out suction catheter clinical trials, in order to bring ADAPT technology advocated in mature markets in Europe and the United States to China.
It is reported that Dr. Wang Jicheng, founder of Wo Mu Biology, once successfully led the company into the top four of coronary artery coated stents as the founder of Jiwei Medical and the former CEO of Biosensors International Group; his team has many years of successful experience in technology research and development and product commercialization, and has obtained a number of patents.

