Exhibit 99.2

生物制药有限公司2024年9月投资者介绍

©2024年,YD BIOPHARMA有限公司免责声明:本投资者介绍(本“介绍”)已经为了潜在的业务组合(“业务组合”)而准备,该业务组合是Breeze Holdings Acquisition Corp.(“SPAC” )和YD生物制药有限公司(“YD生物制药”或“YD BIO”或“目标方”以及与SPAC一起称为“各方”)之间的。本介绍仅供信息目的,并且未经各方事先书面同意,不得全部或部分复制或转发。本介绍并未同时包括或包含可能所需的用于评估业务组合的所有信息。各方保留更新或补充本介绍提供的信息的权利,但不承担任何义务。您不应确切依赖它或将其用作任何与业务组合或其他事项有关的任何决定、合同、承诺或行动的确切依据。您应咨询自己的法律、监管、税务、商业、金融和会计顾问,至于你认为必要的程度,并且你必须做出自己的投资决策,并对各方和业务组合进行自己独立的调查和分析。在法律允许的最大范围内,任何情况下,任一方或其各自的关联公司、官员、董事、雇员、代表、顾问或代理商对于使用本介绍、其内容、其遗漏、依赖其内含信息或针对相关信息传达的意见所引起的任何直接、间接或后果性损失或利润损失概不负责或承担任何责任。本介绍中的信息属高度机密。本介绍应仍然是各方的财产,将本介绍分发或披露给任何其他人是未经授权的,除了您的随从、官员、董事、雇员、代表、顾问或代理商,他们有需要接收此介绍来评估业务组合,并已被告知并同意遵守本处所述的保密义务。未经授权的披露、转发、复制或复印,或本介绍或其任何部分的内容的更改是被禁止的。您应保密保持本介绍及其内容,不得将本介绍或其内容用于任何目的,而非明确得到各方授权,并且应在各方要求后立即归还或销毁您拥有的本介绍副本或其中的部分。通过接受本介绍,您被视为同意前述保密要求。本介绍的任何未经授权的分发或复制任何部分可能导致违反1933年修正案的《美国证券法》。任何一方或其各自的关联公司、高管、董事、雇员、代表、顾问或代理明示或暗示都不担保或陈述与本介绍中包含的信息的准确性或完整性有关,或在此期间提供的任何口头信息、产生的任何数据,或已经或可能根据本介绍所述或暗示的条款或方式效率进行任何交易,或关于未来预测、管理目标、估计、前景或回报的达成或合理性,如果有的话,各方对任何此类信息不承担任何责任、义务或责任(无论是直接还是间接的、合同上的、侵权或其他形式)。各方及其各自的关联公司、高管、董事、雇员、代表、顾问和代理明确否认可能基于本介绍及其中的任何错误或遗漏引起的任何责任。本介绍及其中包含的信息并不构成:(a)(i)就业务组合所涉证券的任何全权代理、同意或授权的征求意见或(ii)出售或交换的任何一方,或出售或交换的征求意见。购买或交换任何证券、商品或工具或相关衍生品的征求意见,也不得在任何未在注册或合格的任何该等司法管辖区的证券法下(b)一项销售证券、商品或工具或相关衍生品的促销或交换将是不合法的,或(b)贷款、联合产权或安排融资、承销或购买或担任代理商或建议者,或以其他任何形式进行任何交易、或承诺资本,或参与任何交易战略。您不应将本介绍的内容解释为法律、监管、税务、会计或投资建议或推荐。我们建议您寻求独立第三方法律、监管、商业、金融、会计和税务建议,以了解本介绍的内容。本介绍不构成任何一方或其各自的关联公司、高管、董事、雇员、代表、顾问或代理商的任何形式的金融或公平意见或建议。这不是研究报告。通过接受本介绍,您确认您不依赖本介绍中包含的信息来做出任何决定。本介绍仅分发给合理相信具有足够专业知识以理解业务组合风险的人士。您应确定经济风险和优缺点,以及法律、监管、商业、金融、会计和税务特性和业务组合的相关风险和自愿决定您能够承担与前述有关的风险。通过接受本介绍,您确认已被告知(a)任何一方都没有为您提供任何法律、监管、商业、金融、会计或税务建议,(b)您了解与业务组合有法律、监管、商业、金融、会计和税务风险,(c)您应该从具有适当专门知识的顾问那里获得法律、监管、商业、金融、会计和税务建议,以评估相关风险和(d)您应告知您组织中的高级管理层与业务组合相关的法律、监管、商业、金融、会计和税务建议(如适用)和有关这些事项的免责声明 。您确认您不依赖本介绍中包含的信息做出任何决定。美国证券交易委员会(“SEC”)或任何州或地方证券委员会或任何其他监管机构尚未批准或不批准本介绍或确认其真实或完整。任何与此相反的陈述都是一种刑事犯罪。

©2024, YD BIOPHARMA ., LTD. Disclaimer YD BIOPHARMA Forward - Looking Statements This Presentation contains certain “forward - looking statements” within the meaning of the United States Private Securities Litigation Reform Act of 1995 , Section 27 A of the Act and Section 21 E of the Securities Exchange Act of 1934 , as amended . All statements other than statements of historical fact contained in this Presentation, including, but not limited to, statements as to the transactions contemplated by the Business Combination and related agreements, the benefits or timing of the Business Combination, the effects of regulations, projected future results of operations and financial position, revenue and other metrics, planned products and services, business strategy and plans, objectives of management for future operations, market size and growth opportunities, competitive position and technological and market trends, are forward - looking statements . Some of these forward - looking statements can be identified by the use of forward - looking words, including, but not limited to, “may,” “should,” “expect,” “intend,” “will,” “estimate,” “anticipate,” “believe,” “predict,” “plan,” “targets,” “projects,” “could,” “would,” “continue,” “forecast,” “strategy,” and “opportunity” or the negatives of these terms or variations of them or similar expressions . All forward - looking statements are subject to risks, uncertainties, and other factors (including those which are beyond the control of either Party) which could cause actual results to differ materially from those expressed or implied by such forward - looking statements . All forward - looking statements are based upon estimates, forecasts and assumptions that, while considered reasonable by the Parties, are inherently uncertain, and many factors may cause the actual results to differ materially from current expectations, which include, but are not limited to : (a) the occurrence of any event, change or other circumstances that could give rise to the termination of the definitive agreements executed by the Parties with respect to the Business Combination ; (b) the outcome of any legal proceedings that may be instituted against either Party or the combined company, or any of their respective directors or officers, following the announcement of the Business Combination and the transactions contemplated thereby ; (c) the inability to complete the Business Combination due to the failure to obtain approval of the stockholders of either Party, or to satisfy other conditions to closing of the Business Combination ; (d) changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining applicable regulatory approvals for the Business Combination ; (e) the ability to meet or maintain the Nasdaq Stock Market’s listing standards prior to or following the consummation of the Business Combination ; (f) the risk that the announcement or consummation of the Business Combination disrupts current plans and operations of either Party ; (g) the inability to recognize the anticipated benefits of the Business Combination ; (h) the ability of the combined company to successfully increase market penetration into its target markets, to execute on its business strategy or to compete effectively ; (i) the addressable markets that the Parties intend to target do not grow as expected ; (j) the loss of any key executives ; (k) the loss of any relationships with key suppliers or customers (as may be applicable) or the inability to attract and retain customers ; (l) the inability to protect patents and other intellectual property ; (n) costs related to the Business Combination ; (o) changes in applicable laws or regulations ; (p) the possibility that either Party or the combined company may be adversely affected by other economic, business and/or competitive factors ; (q) the Parties’ estimates of growth and projected financial results and meeting or satisfying the underlying assumptions with respect thereto ; (r) the risk that the Business Combination may not be completed in a timely manner or at all, which may adversely affect the price of either Party’s securities ; (s) the risk that the transaction may not be completed by SPAC’s business combination deadline (as may be extended pursuant to SPAC’s governing documents) ; (t) the impact of the novel coronavirus disease pandemic, including any mutations or variants thereof, and its effect on business and financial conditions ; and (u) other risks and uncertainties set forth in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward - Looking Statements” in SPAC’s Form S - 1 (File No . 333 - 249677 ), annual report on Form 10 - K/A for the year ended December 31 , 2023 , quarterly report on Form 10 - Q for the period ended June 30 , 2024 and, when available, the registration statement on Form F - 4 to be filed with the SEC in connection with the Business Combination, which will include a document that serves as a preliminary prospectus and proxy statement of SPAC, referred to as a proxy statement/prospectus, and other documents filed or to be filed from time to time with the SEC in connection with the Business Combination . The foregoing list is not exhaustive, and new risks may emerge from time to time . These filings identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward - looking statements . Nothing in this Presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward looking statements will be achieved . You should not place undue reliance on forward - looking statements, which speak only as of the date they are made . Neither Party, nor any of their respective affiliates, gives any assurance that the combined company will achieve its currently expected results . Neither Party nor the combined company undertakes any duty to update or revise these forward - looking statements, except as otherwise required by law . Use of Projections This Presentation may contain financial forecasts of the Parties and the combined company which are based on currently available information and estimates . Neither Party nor its independent auditors, nor the independent registered public accounting firm of either Party, has audited, reviewed, compiled or performed any procedures with respect to the projections for the purpose of their inclusion in this Presentation, and accordingly, none of the foregoing has expressed an opinion or provided any other form of assurance with respect thereto for the purpose of this Presentation . These projections are forward - looking statements and should not be relied upon as being necessarily indicative of future results . The projected financial information contained in this Presentation constitutes forward - looking information, and neither Party nor the combined company undertakes any duty to update or revise the projected financial information . The assumptions and estimates underlying such projected financial information are inherently uncertain and are subject to a wide variety of significant business, economic, competitive, and other risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information . See the section titled “Forward - Looking Statements” above . Accordingly, there can be no assurance that the prospective results are indicative of future performance or that actual results will not differ materially from the results presented in the prospective financial information contained in this Presentation . Actual results may differ materially from the results contemplated by the projected financial information contained in this Presentation . The inclusion of such information in this Presentation should not be regarded as a representation by any person that the results reflected in such projections will be achieved . 3

©2024年, YD BIOPHARMA有限公司。免责声明YD BIOPHARMA财务信息本演示文稿中包含的某些财务信息和数据未经审计,可能不符合规定S-X。这些信息和数据可能不包括在即将提交给SEC与企业合并有关的注册声明中,可能会进行调整或以不同方式呈现。商标每个方拥有或拥有各种商标、服务标记、版权、商业名称和产品的权利,用于与其业务的操作有关。本演示文稿还可能包含其他公司的商标、服务标记、版权、商业名称或产品,这些商标、服务标记、版权、商业名称或产品归其各自所有者所有。在本演示文稿中使用或展示第三方商标、服务标记、版权、商业名称或产品并不意味着Party之间具有关系,也不意味着Party的赞助或支持。仅仅出于便利起见,本演示文稿中提到的商标、服务标记、商业名称和版权可能没有带有Tm、Sm、©或®符号,但这些引用并不打算在任何方面表明,每个方将不维护,根据适用法律的规定,其权利或相应所有者(如果有)对这些商标、服务标记、版权、商业名称和产品的权利。行业和市场数据本演示文稿包含并依赖于从第三方来源和内部来源获取的某些信息。这些信息涉及许多假设和限制;因此,无法保证此类假设的准确性或可靠性,因此,您应该谨慎对此信息给予过多重视。此外,任何一方均不就本文中所作的任何假设的合理性或此处包含的任何预测或任何其他信息的准确性或完整性作出明示或暗示的陈述或担保,并在法律允许的最大范围内,各方特此免除对因使用此类信息而导致的任何直接、间接或间接的损失或利润损失的任何责任。此处包含的有关过去业绩或建模的任何数据并不表示未来表现的指示。任何一方或其各自的关联公司、官员、董事、雇员、代表或顾问均未独立核实任何此类第三方信息的准确性或完整性。同样,Party主题进行的其他第三方调查数据和研究报告虽然被认为是可靠的,但基于有限的样本规模,并未由Parties进行独立核实。此外,对Target运营的行业未来表现以及其未来表现的预测、假设、估计、目标、计划和趋势的投影,由于各种因素,包括以上描述的那些因素,必然会受到不确定性和风险的影响。这些和其他因素可能导致结果与独立方和Party所做估计中表达的结果有重大不同。附加信息及查阅方式Party打算提交SPAC与SEC有关企业组合的一份明确的代理声明/招股说明书(“明确代理声明/招股说明书”)。明确代理声明/招股说明书将发送给SPAC和Target的所有股东,作为一个待建立的记录日期。Party还将向SEC提交有关企业组合的其他文件。在做出任何投票或其他投资决策之前,敦促SPAC和Target的投资者和证券持有人阅读明确的代理声明/招股说明书以及与企业组合有关的所有其他已提交或将提交的有关文件,因为它们将包含有关Party和企业组合的重要信息。投资者和证券持有人可通过SEC维护的网站www.sec.gov免费获取Party在与企业组合有关的文件中已提交或将提交的明确的代理声明/招股说明书和所有其他相关文件副本。通过这样一个网站。招徕征集Party及与企业组合有关的明确协议中的某些其他方,以及他们各自的董事、高管和其他管理人员和雇员,根据SEC规则,可能被视为会参与从SPAC的股东那里征集代理人的活动,以便与企业组合有关。这些人的姓名列表以及有关他们在企业组合中的利益的信息将包含在明确的代理声明/招股说明书中。当可用时,您可以通过向SPAC发送邮件请求免费获得这些文件的副本。此演示文稿未包含应与企业组合有关的所有信息。它不打算形成任何投资决策或有关企业组合的任何决定的任何依据。4

©2024年,YD BIOPHARMA有限公司。 YD BIO与Breeze Holdings Acquisition Corp.合并。 YD BIOPHARMA关键管理人员• SPAC概览:Breeze Holdings Acquisition Corp.(OTCQX:BRZH)是一家公开上市的特殊目的收购公司,大约有1000万美元的现金。• 估值:公司的拟议净资产价值约为70000万美元。• 结构:Breeze和YD BIO将各自合并为新成立的开曼控股公司的全资子公司,预计将命名为“YD Bio Limited”。合并后,YD Bio Limited预计将在纳斯达克上市。• 所有权:现有的YD BIO股东:90.6%,PIPE股东:2.6%,SPAC公开股份:2.1%,SPAC内部股份:4.7%。• 时间:预计交易将于25年第1季度结束,视常规收盘条件和任何必要的监管批准而定。• 管理和董事会构成:交易完成后,YD BIO将继续由沈峰博士领导。董事会将由YD BIO的CEO,YD BIO指定的五(5)名董事和Breeze Holdings指定的两(2)名董事组成。 Breeze和YD生物制药公司的合作结合了上市公司经验和行业专业知识交易详情经验名称及职务〜25年 道格·拉姆齐博士 主席及首席执行官 〜35年 Russ Griffin 董事 及 总裁 〜23年 沈峰博士 主席及首席执行官 〜17年 吴承峰 首席医务官 〜35年 蔡美玲 首席业务官 〜18年 陈吉恩 会计经理 Breeze Holdings YD生物制药公司5
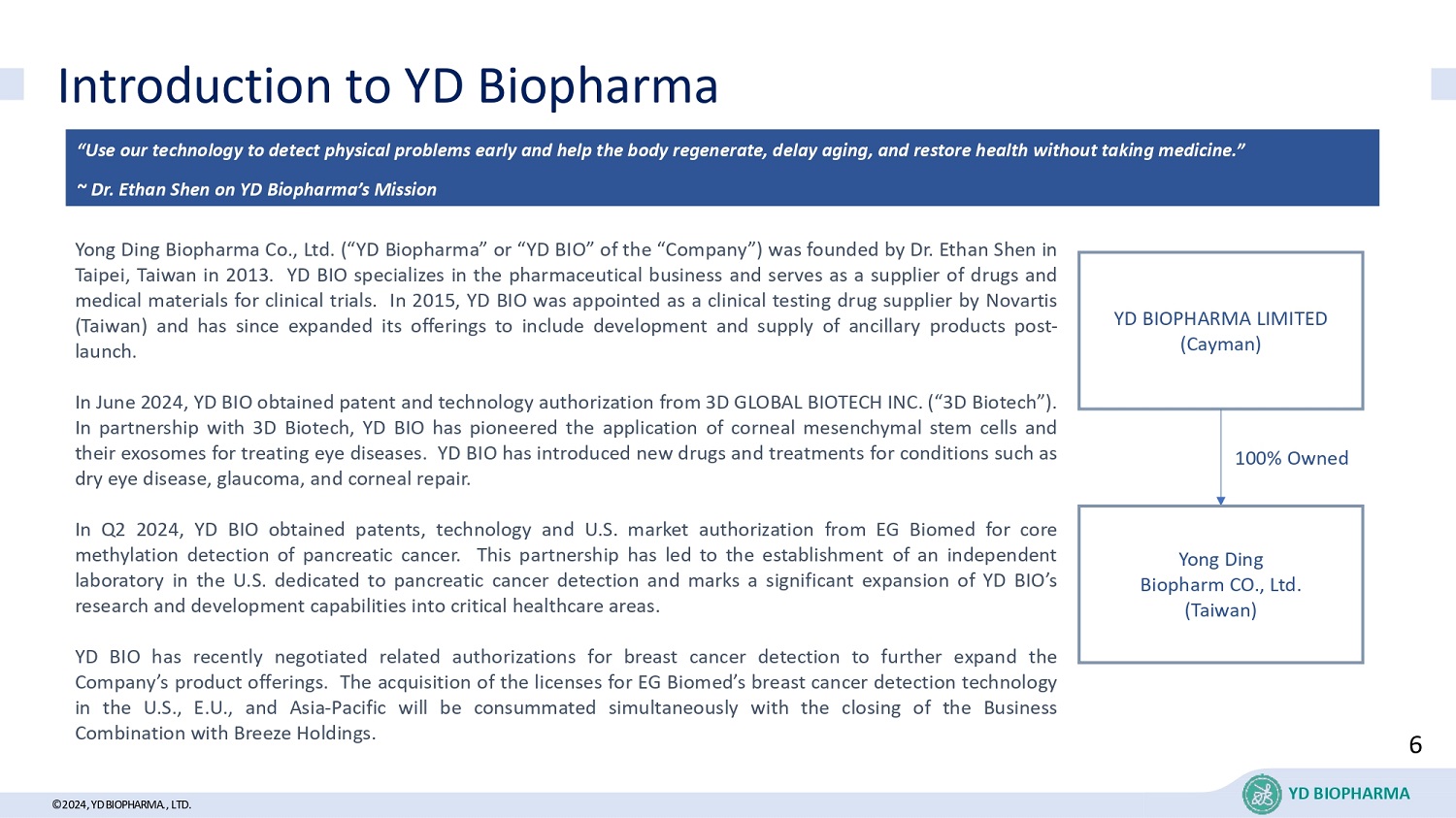
©2024年,远东生物医药有限公司介绍:远东生物医药永鼎生物医药股份有限公司(以下简称“远东生物医药”或“YD BIOPHARMA”或“YD BIO”,公司)由沈伊桐博士于2013年在台湾台北创立。远东生物医药专注于医药商业,是临床试验药物和医疗材料供应商。2015年,远东生物医药被诺华(台湾)任命为临床试验药物供应商,并自那时起扩展其产品线以包括上市后的附属产品的开发和供应。2024年6月,远东生物医药从3D GLOBAL生物技术公司(“3D Biotech”)获得了专利和技术授权。与3D Biotech合作,远东生物医药引领了角膜间充质干细胞及其外泌体治疗眼部疾病的应用。远东生物医药推出了针对干眼症、青光眼和角膜修复等病症的新药物和治疗方法。2024年第二季度,远东生物医药从EG Biomed获得了胰腺癌核甲基化检测的专利、技术和美国市场授权。这一合作导致在美国建立了一个专门用于胰腺癌检测的独立实验室,并标志着远东生物医药将其研发能力拓展到重要医疗领域的重大扩张。远东生物医药最近就乳腺癌检测进行了相关授权谈判,以进一步扩展公司的产品线。在与Breeze Holdings的业务合并关闭交易同时,远东生物医药将同时完成对EG Biomed的乳腺癌检测技术在美国、欧盟和亚太地区的许可收购。沈伊桐博士关于远东生物医药使命的观点:“利用我们的技术及早发现身体问题,并帮助身体再生、延缓衰老、恢复健康,无需服用药物。”YD BIOPHARMA LIMITED(开曼群岛)永鼎生物医药股份有限公司(台湾)全资拥有
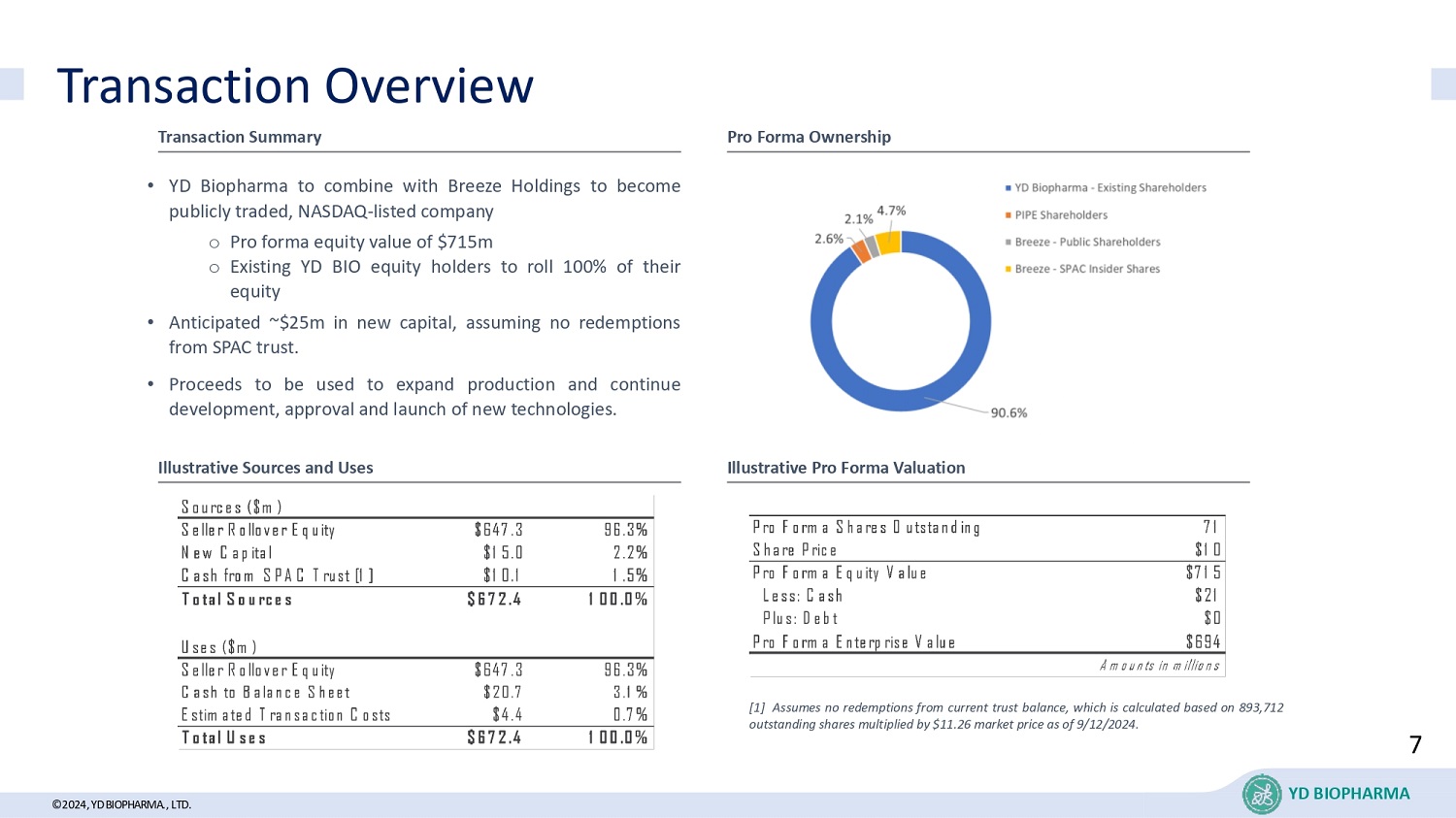
©2024, YD BIOPHARMA ., LTD. Transaction Overview YD BIOPHARMA Transaction Summary Pro Forma Ownership Illustrative Sources and Uses Illustrative Pro Forma Valuation • YD Biopharma to combine with Breeze Holdings to become publicly traded, NASDAQ - listed company o Pro forma equity value of $ 715 m o Existing YD BIO equity holders to roll 100 % of their equity • Anticipated ~ $ 25 m in new capital, assuming no redemptions from SPAC trust . • Proceeds to be used to expand production and continue development, approval and launch of new technologies . 95.4% Pro Forma Shares Outstanding 71 Share Price $10 Pro Forma Equity Value $715 Less: Cash $21 Plus: Debt $0 Pro Forma Enterprise Value $694 Amounts in millions [ 1 ] Assumes no redemptions from current trust balance, which is calculated based on 893 , 712 outstanding shares multiplied by $ 11 . 26 market price as of 9 / 12 / 2024 . Sources ($m) Seller Rollover Equity $647.3 96.3% New Capital $15.0 2.2% Cash from SPAC Trust [1] $10.1 1.5% Total Sources $672.4 100.0% Uses ($m) Seller Rollover Equity $647.3 96.3% Cash to Balance Sheet $20.7 3.1% Estimated Transaction Costs $4.4 0.7% Total Uses $672.4 100.0% 7
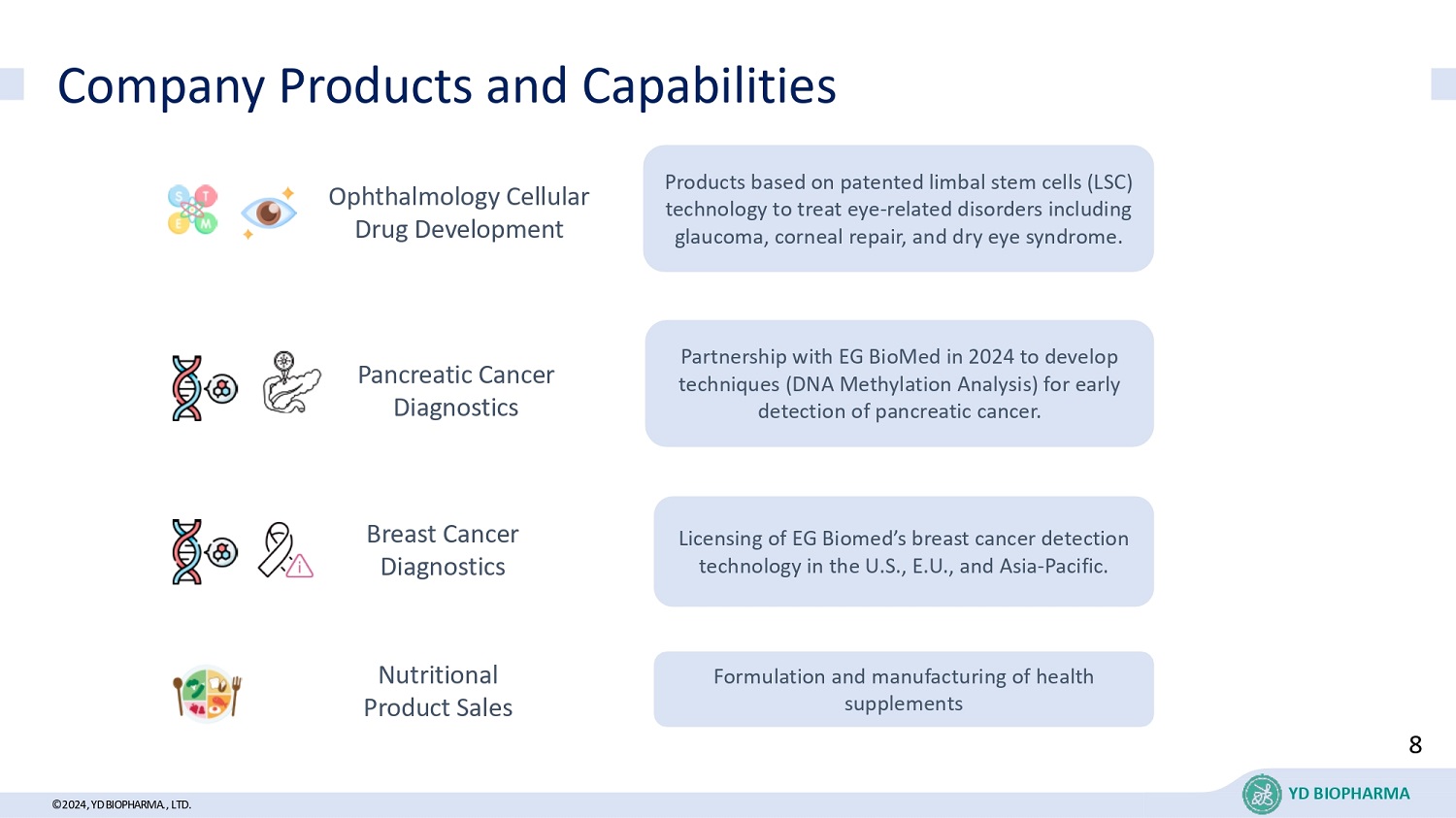
©2024, YD BIOPHARMA ., LTD. Company Products and Capabilities YD BIOPHARMA 8 Formulation and manufacturing of health supplements Products based on patented limbal stem cells (LSC) technology to treat eye - related disorders including glaucoma, corneal repair, and dry eye syndrome. Partnership with EG BioMed in 2024 to develop techniques (DNA Methylation Analysis) for early detection of pancreatic cancer. Licensing of EG Biomed’s breast cancer detection technology in the U.S., E.U., and Asia - Pacific. Pancreatic Cancer Diagnostics Breast Cancer Diagnostics Ophthalmology Cellular Drug Development Nutritional Product Sales
©2024, YD BIOPHARMA ., LTD. Strategic Partnerships YD BIOPHARMA YD Biopharma appointed a clinical testing drug supplier by Novartis (Taiwan) in 2015. Licensing partnership with 3D Global Biotech in 2024 to develop products based on patented limbal stem cells (LSC) technology for the treatment of eye disorders such as glaucoma, corneal repair, and dry eye syndrome. 3D Global Biotech is publicly - traded on the Taipei Stock Exchange. Licensing partnership with EG BioMed, a biomedical and healthcare startup in Taiwan developing new technologies for cancer detection. YD BIO acquired authorization to use EG Biomed’s pancreatic cancer detection technology in Q2 2024 and is finalizing the licensing agreement for EG Biomed’s breast cancer detection technology in the U.S., E.U., and Asia - Pacific. 9
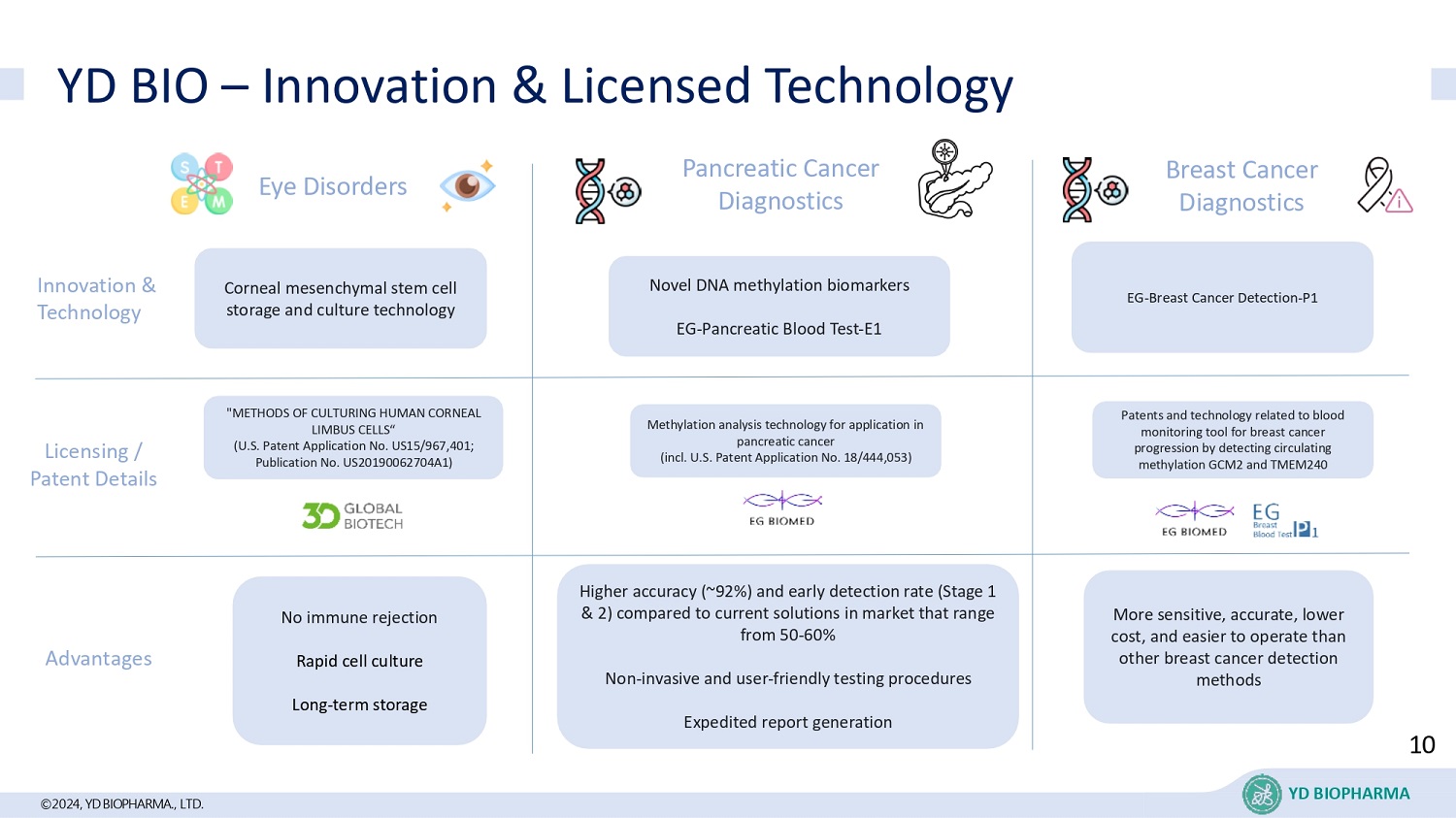
©2024, YD BIOPHARMA ., LTD. YD BIO – Innovation & Licensed Technology Eye Disorders No immune rejection Rapid cell culture Long - term storage Pancreatic Cancer Diagnostics Higher accuracy (~92%) and early detection rate (Stage 1 & 2) compared to current solutions in market that range from 50 - 60% Non - invasive and user - friendly testing procedures Expedited report generation Breast Cancer Diagnostics More sensitive, accurate, lower cost, and easier to operate than other breast cancer detection methods Licensing / Patent Details Advantages Novel DNA methylation biomarkers EG - Pancreatic Blood Test - E1 Corneal mesenchymal stem cell storage and culture technology EG - Breast Cancer Detection - P1 Innovation & Technology "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS“ (U.S. Patent Application No. US15/967,401; Publication No. US20190062704A1) Patents and technology related to blood monitoring tool for breast cancer progression by detecting circulating methylation GCM2 and TMEM240 Methylation analysis technology for application in pancreatic cancer (incl. U.S. Patent Application No. 18/444,053) 10 YD BIOPHARMA
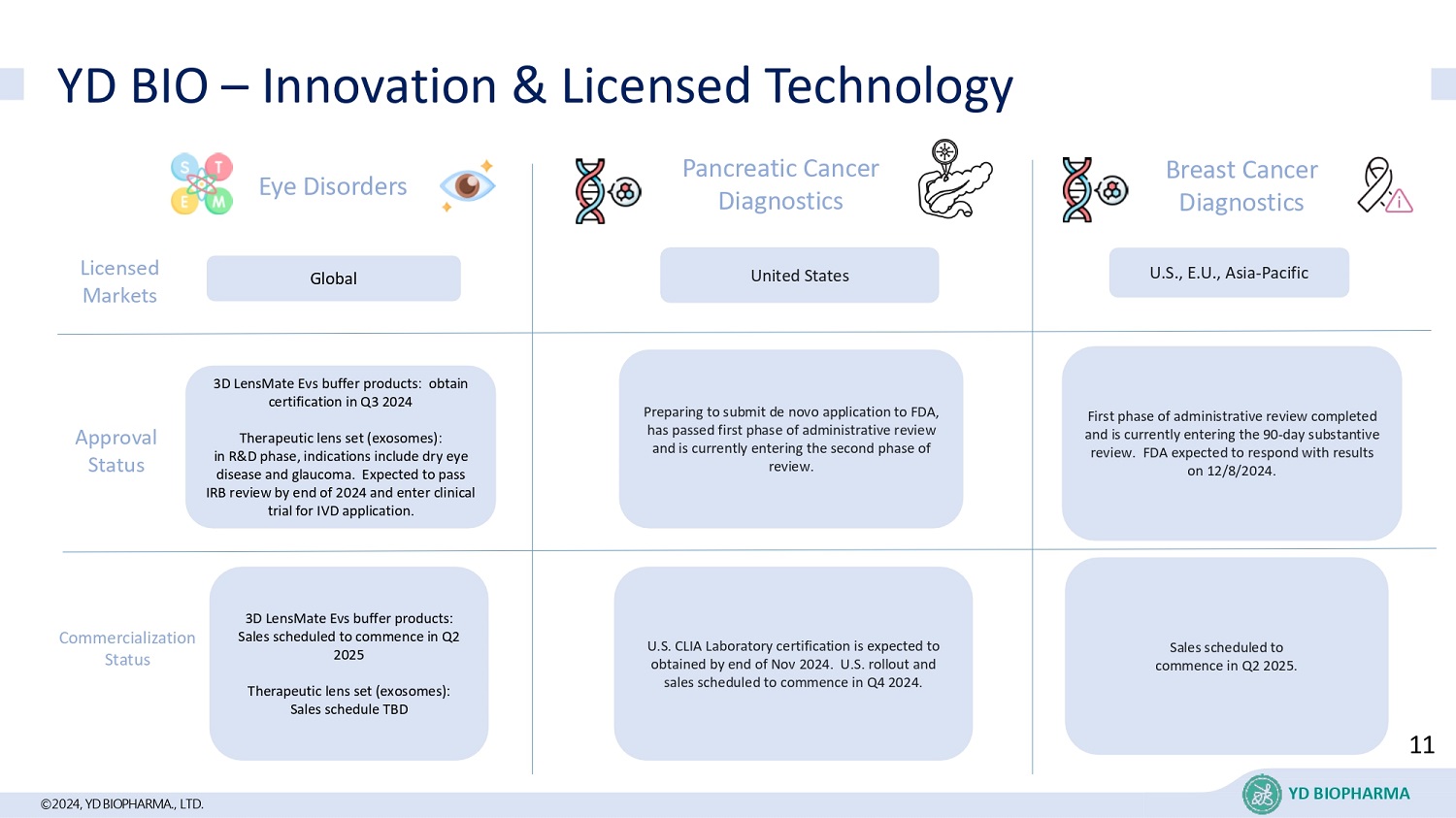
©2024, YD BIOPHARMA ., LTD. YD BIO – Innovation & Licensed Technology Global Pancreatic Cancer Diagnostics United States Breast Cancer Diagnostics U.S., E.U., Asia - Pacific L icensed Markets Approval Status 3D LensMate Evs buffer products: obtain certification in Q3 2024 Therapeutic lens set (exosomes): in R&D phase, indications include dry eye disease and glaucoma. Expected to pass IRB review by end of 2024 and enter clinical trial for IVD application. First phase of administrative review completed and is currently entering the 90 - day substantive review. FDA expected to respond with results on 12/8/2024. Preparing to submit de novo application to FDA, has passed first phase of administrative review and is currently entering the second phase of review. Commercialization Status U.S. CLIA Laboratory certification is expected to obtained by end of Nov 2024. U.S. rollout and sales scheduled to commence in Q4 2024. Sales scheduled to commence in Q2 2025. 3D LensMate Evs buffer products: Sales scheduled to commence in Q2 2025 Therapeutic lens set (exosomes): Sales schedule TBD Eye Disorders 11 YD BIOPHARMA
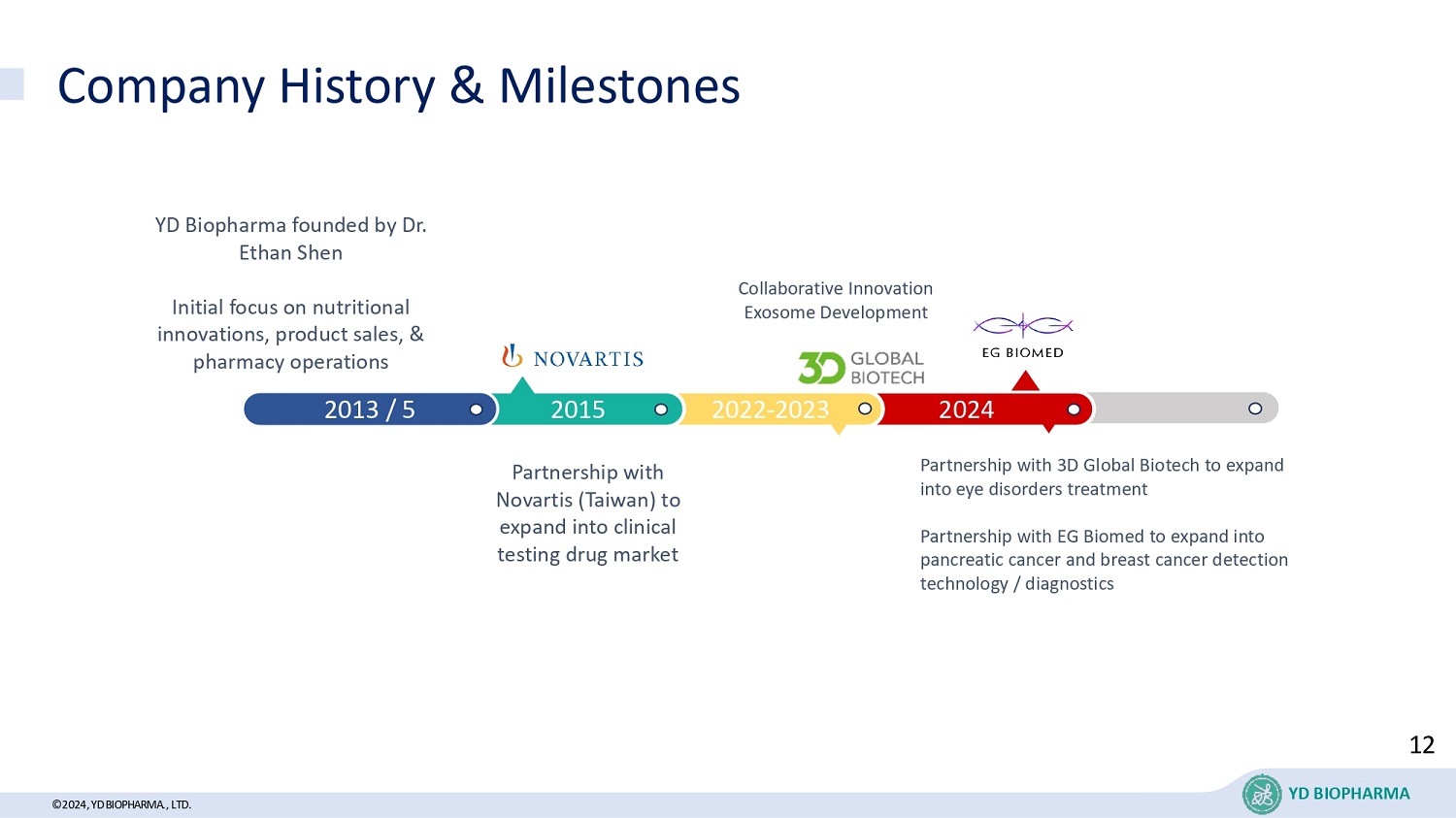
©2024, YD BIOPHARMA ., LTD. Company History & Milestones YD BIOPHARMA YD Biopharma founded by Dr. Ethan Shen Initial focus on nutritional innovations, product sales, & pharmacy operations Partnership with Novartis (Taiwan) to expand into clinical testing drug market Collaborative Innovation Exosome Development Partnership with 3D Global Biotech to expand into eye disorders treatment Partnership with EG Biomed to expand into pancreatic cancer and breast cancer detection technology / diagnostics 2024 2022 - 2023 2015 2013 / 5 12
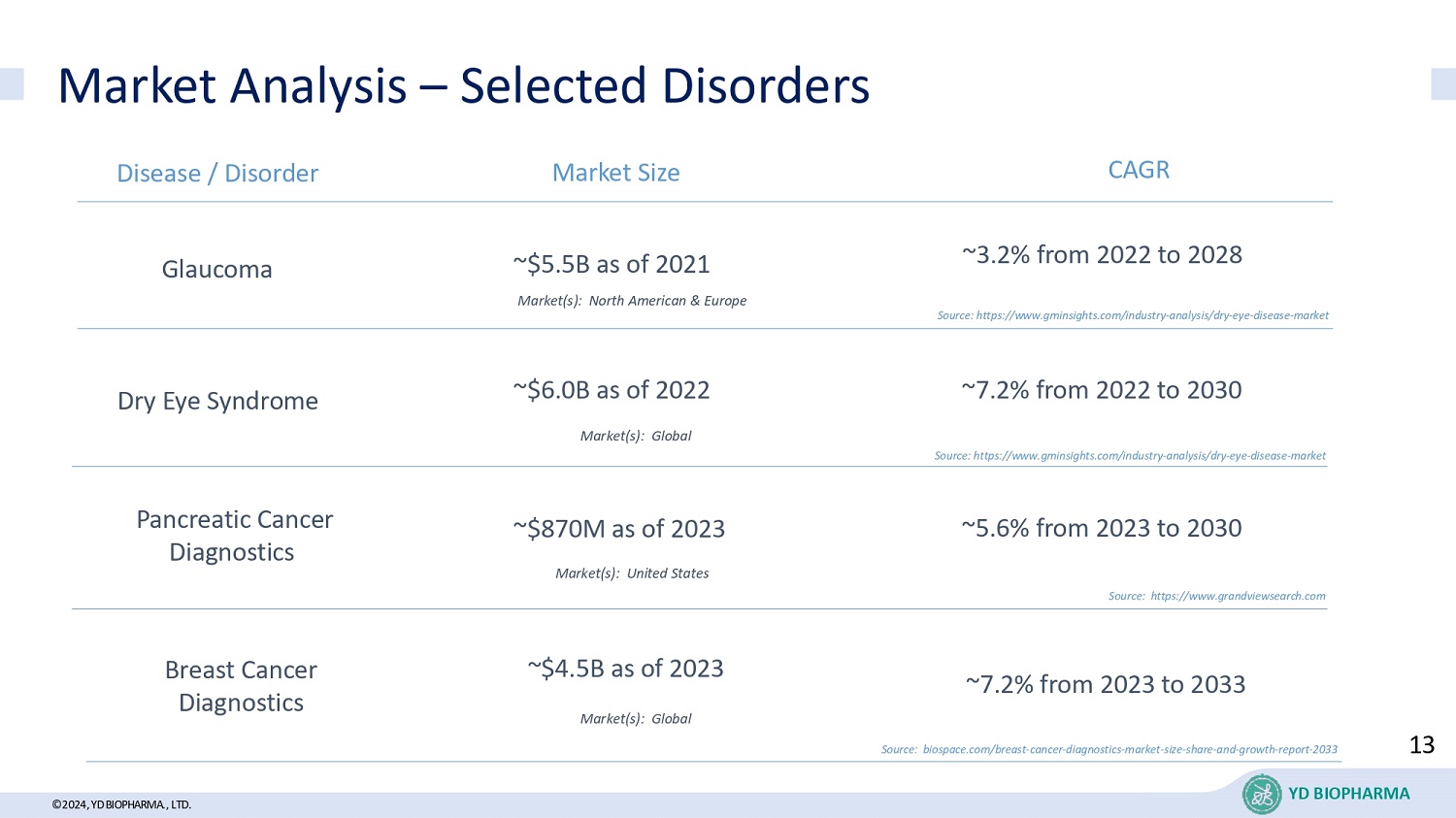
©2024, YD BIOPHARMA ., LTD. Market Analysis – Selected Disorders YD BIOPHARMA Pancreatic Cancer Diagnostics Glaucoma Dry Eye Syndrome Disease / Disorder Market Size ~$870M as of 2023 CAGR ~5.6% from 2023 to 2030 ~$5.5B as of 2021 ~3.2% from 2022 to 2028 ~$6.0B as of 2022 Source: https://www.grandviewsearch.com ~7.2% from 2022 to 2030 Source: https://www.gminsights.com/industry - analysis/dry - eye - disease - market Breast Cancer Diagnostics ~$4.5B as of 2023 ~7.2% from 2023 to 2033 Source: biospace.com/breast - cancer - diagnostics - market - size - share - and - growth - report - 2033 Market(s): Global Market(s): North American & Europe Source: https://www.gminsights.com/industry - analysis/dry - eye - disease - market Market(s): Global Market(s): United States 13
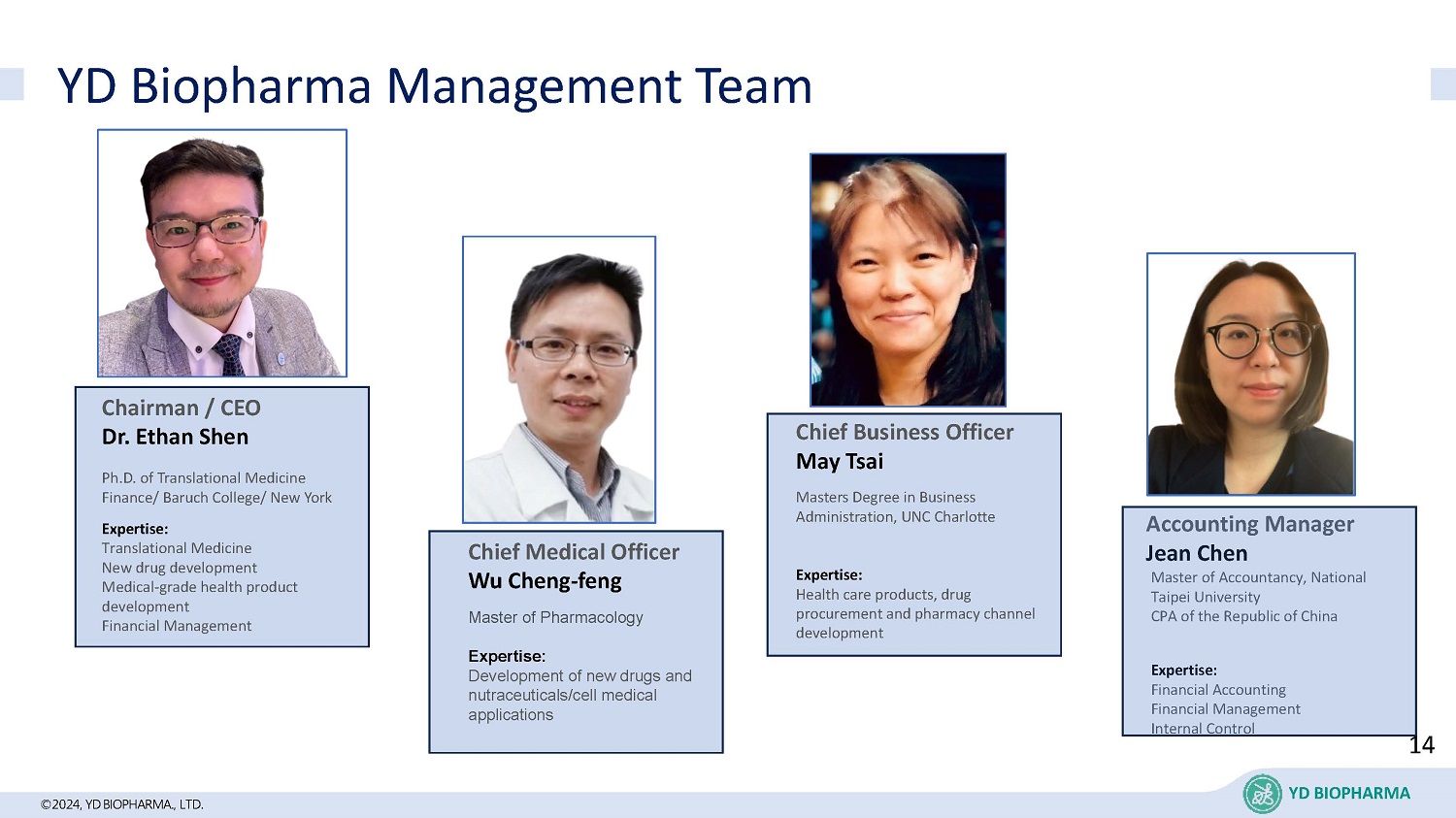
©2024, YD BIOPHARMA ., LTD. YD Biopharma Management Team Ph.D. of Translational Medicine Finance/ Baruch College/ New York Expertise: Translational Medicine New drug development Medical - grade health product development Financial Management Chairman / CEO Dr. Ethan Shen C hief Medical Officer Wu Cheng - feng Master of Pharmacology Expertise: Development of new drugs and nutraceuticals/cell medical applications C hief Business Officer May Tsai Masters Degree in Business Administration, UNC Charlotte Expertise: H ealth care products, drug procurement and pharmacy channel development Master of Accountancy, National Taipei University CPA of the Republic of China Expertise: Financial Accounting Financial Management Internal Control Accounting Manager Jean Chen 14 YD BIOPHARMA

Doug Ramsey, Ph.D. Chairman, CEO, CFO and Director ▪ Finance professor at SMU, Baylor and Cal Poly Pomona ▪ BS in Finance from Cal Poly Pomona, MBA from the University of Chicago Booth School of Business and an MA and Ph.D. in Business and Financial Economics from Claremont Graduate University ▪ Public - company CFO experience ▪ National Association of Corporate Directors (NACD) – Directorship Certified ©2024, YD BIOPHARMA ., LTD. Breeze Holdings Acquisition Corp. Management Team – Selected Highlights Russ Griffin President and Director ▪ BS in Petroleum Engineering Technology from Nicholls State University ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international ▪ National Association of Corporate Directors (NACD) – Directorship Certified Charles Ross Chief Operating Officer ▪ BS in Engineering from UT Austin ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international Aaron Ortega Executive VP of Business Development ▪ BA from Duke University, and an MBA from Southern Methodist University’s Cox School of Business ▪ Led or participated in multiple acquisitions and divestitures, both domestic and international SPAC Board Members: Albert McLelland, Robert Thomas, Bill Stark and Gen. James Williams. Various Roles & Credentials ▪ Director of the Chairman’s Asian Cross - Border Transactions Initiative for PwC ▪ Adjunct Professor at SMU Caruth Institute for Entrepreneurship in the Cox School of Business ▪ Extensive international operating, capital markets and corporate governance experience including multiple director roles on b eha lf of the Carlyle Group and other prominent financial services firms 15 YD BIOPHARMA
©2024, YD BIOPHARMA ., LTD. Biotech Sector Strength – Recent Transactions YD BIOPHARMA MBX Biosciences (Nasdaq: MBX) Bicara Therapeutics (Nasdaq: BCAX) Zenas BioPharma (Nasdaq: ZBIO) A developer of therapies for endocrine and metabolic disorders, MBX shares traded immediately post - IPO up 48 % to over $ 23 /share (above its IPO price of $ 16 /share) . MBX’s upsized IPO raised $ 163 M, pricing 10 . 2 M shares at the upper limit of the price range . Order book was reportedly 7 x oversubscribed . A provider of treatments targeting head and neck carcinomas, BCAX shares traded immediately post - IPO up 30 % to over $ 23 /share . BCAX upsized its IPO to sell 17 . 5 M shares at $ 18 (at the top of the price range) to raise $ 315 M . Order book was reportedly 10 x oversubscribed . A number of successful IPOs in the biotech sector have priced in September 2024, representing the busiest period for biotech and pharmaceutical IPOs since July 2021. A developer of therapies for patients with autoimmune diseases, ZBIO shares traded immediately post - IPO up 6 . 8 % to over $ 18 /share . The IPO raised $ 225 M priced at $ 17 /share (the middle of the marketed range) . 16

APPENDIX

We will strive to surpass our competitors within two years Galleri EGPT - E1 Test Grail EG BIOMED Company NGS platform qPCR platform platform High Low Testing Cost Analysis takes a long time and reporting is slow Short analysis time and quick report generation Test of Time High construction costs Unable to quickly deploy globally Low construction cost Rapid global layout Global layout Through low detection cost, fast speed and low construction cost, we hope to surpass our competitors in 2 years . 18 YD BIOPHARMA
©2024, YD BIOPHARMA ., LTD. Publications Related to Licensed Technology 19 YD BIOPHARMA
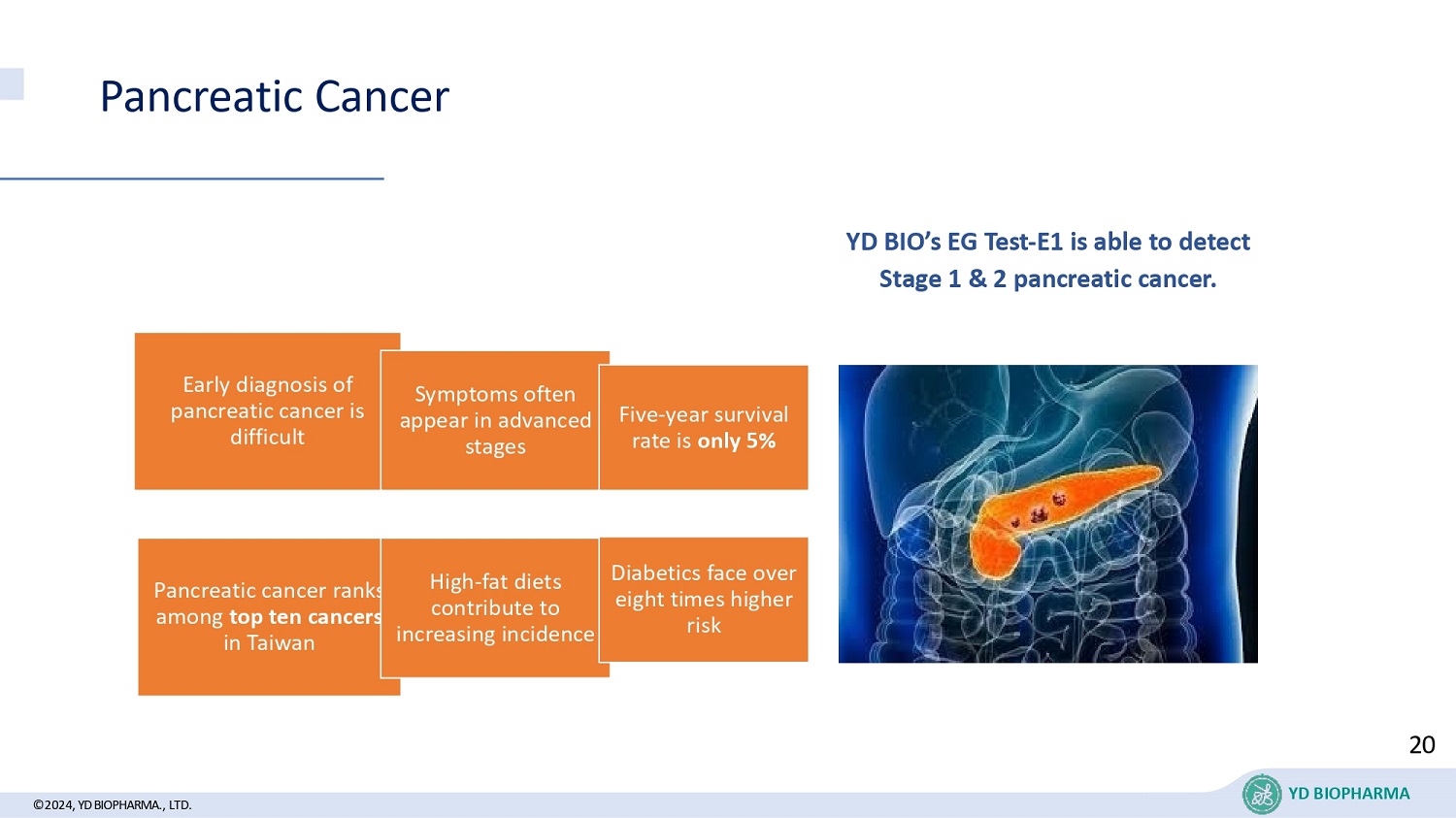
Pancreatic cancer ranks among top ten cancers in Taiwan Pancreatic Cancer ©2024, YD BIOPHARMA ., LTD. Early diagnosis of pancreatic cancer is difficult Symptoms often appear in advanced stages Five - year survival rate is only 5% High - fat diets contribute to increasing incidence Diabetics face over eight times higher risk YD BIO’s EG Test - E1 is able to detect Stage 1 & 2 pancreatic cancer. 20 YD BIOPHARMA
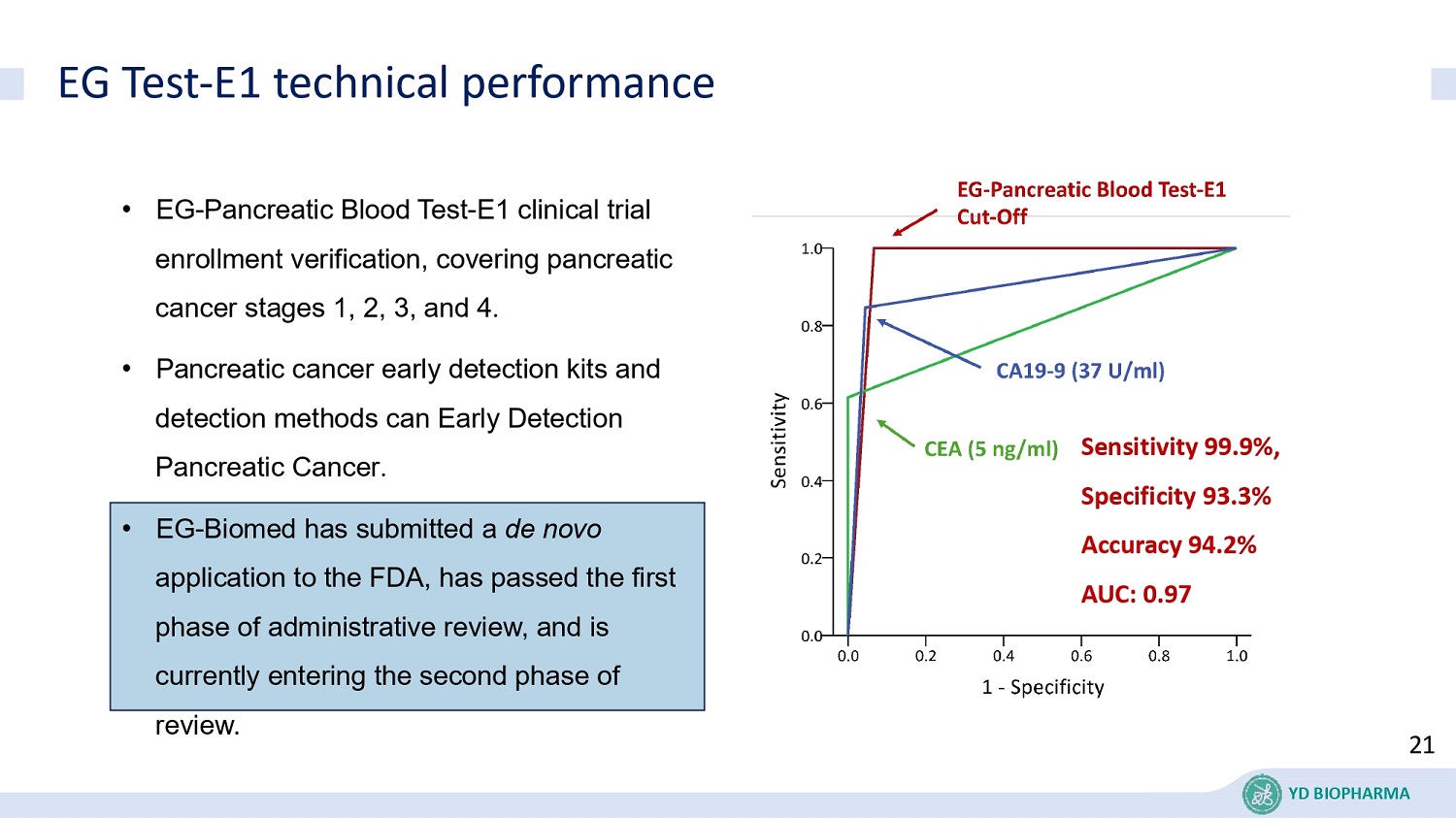
EG Test - E1 technical performance • EG - Pancreatic Blood Test - E1 clinical trial enrollment verification, covering pancreatic cancer stages 1, 2, 3, and 4 . • Pancreatic cancer early detection kits and detection methods can Early Detection Pancreatic Cancer . • EG - Biomed has submitted a de novo application to the FDA, has passed the first phase of administrative review, and is currently entering the second phase of review. Sensitivity 99.9%, Specificity 93.3% Accuracy 94.2% AUC: 0.97 21 YD BIOPHARMA

Breast cancer diagnosis requires invasive and anesthetic breast biopsy procedures, which can be stressful for patients. Current blood tests for breast cancer monitoring lack sufficient sensitivity . The Clinical Need for Precise, Non - Invasive Blood Cancer Detection Breast Cancer 22 ©2024, YD BIOPHARMA ., LTD. YD BIOPHARMA
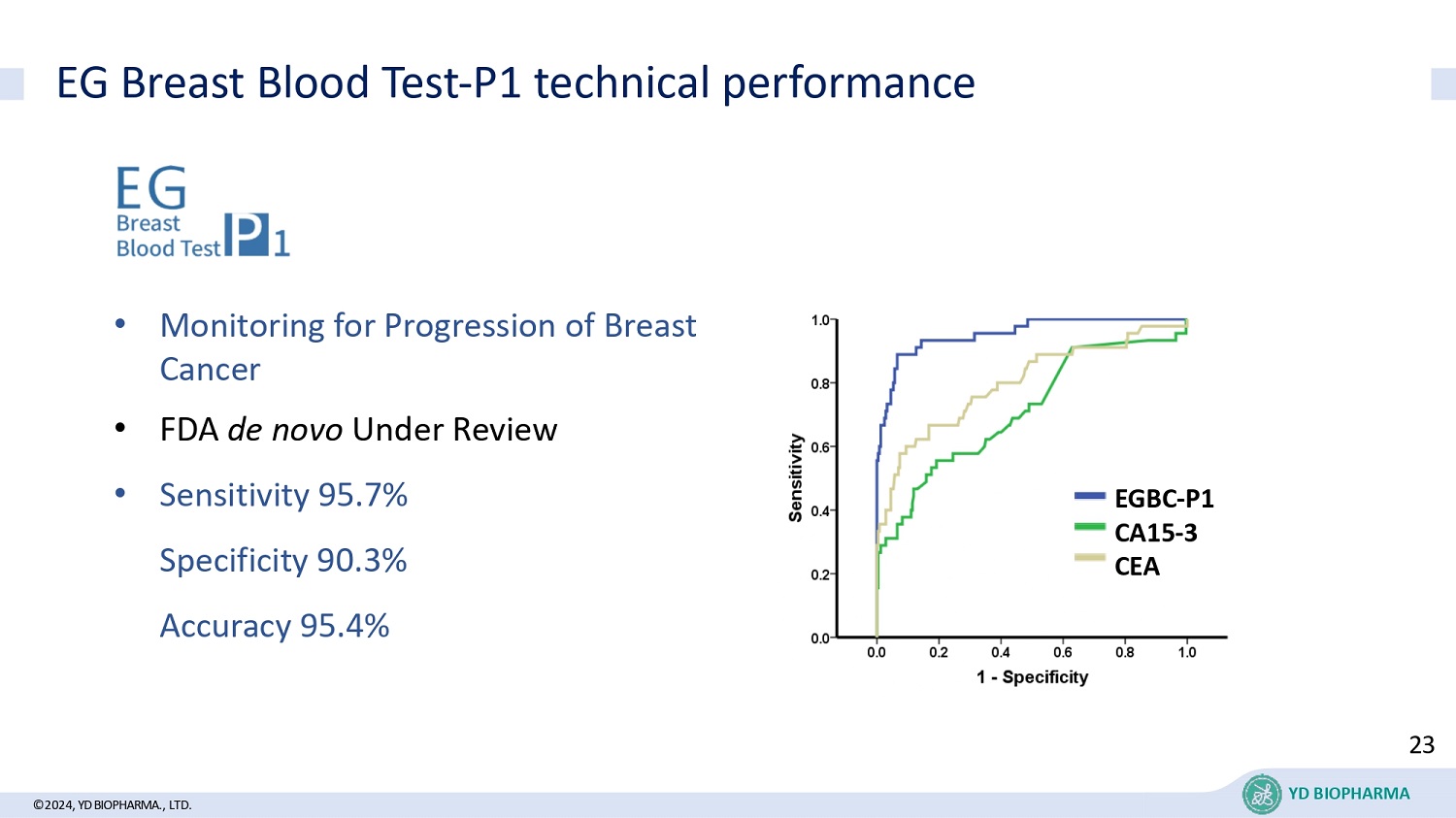
EG Breast Blood Test - P1 technical performance • Monitoring for Progression of Breast Cancer • FDA de novo Under Review • Sensitivity 95.7% Specificity 90.3% Accuracy 95.4% EGBC - P1 CA15 - 3 CEA 23 ©2024, YD BIOPHARMA ., LTD. YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement from 3D GLOBAL BIOTECH INC. • Licensed Patent and Know - How: "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS" (U.S. Patent Application No. US15/967,401; Publication No. US20190062704A1), and owns the relevant know - how and technical data. • Scope of License: 3D GLOBAL 's Patent and Know - How, as well as its know - how in the technology of cell culture process, technology of cell bank construction , exosome purification and authentication technology, and exosome production , which are related to and/or derived from "METHODS OF CULTURING HUMAN CORNEAL LIMBUS CELLS" . YD Biopharma obtains a global, exclusive license from 3D GLOBAL to use the licensed subject to develop, manufacture, offer for sale, sell, use, or import for the above purpose the Product. • Method of License: Global, exclusive license. and agrees YD Biopharma can sublicense to a third party. » Authorization tim e: From the effective date of this Agreement until the expiration of twenty (20) years after all the Product have/has been put on the market. » License Fees and Royalties from selling the Product: Total Licensing fee is USD 5,000,000, Signature Deposit is USD 1,000,0000, Other USD4,000,000 authorization fees will be paid upon milestone completion. and a royalty of 10% of the " Gross Sales" of the Product on a quarterly basis. » Sub - licenses Fee: 20% of the sub - license fees 24 YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement (Pancreatic cancer Detection) from EG BioMed • Licensed Patent and Know - How: methylation analysis technology for application in pancreatic cancer (including U.S. Patent Application No. 18/444,053) and the relevant know - how, and agrees to have the aforesaid patent(s) and relevant know - how • Scope of License: use of the Licensed Patent and Know - How in the Licensed Territory (United States) for manufacturing, offering for sale, selling, using, or importing the Product for the aforementioned purposes. High - fat diets contribute to increasing incidence • Method of License: E xclusive license in U.S. » Authorization tim e : 10 years and will be automatically renewed for another five years or until the expiration of the Licensed Patent » License Fees and Royalties from selling the Product: NT$60,000,000 (tax excluded) and a royalty of 7% of the " Gross Sales" of the Product on a quarterly basis. 25 YD BIOPHARMA

Summary of Patent Licensing and Technology Transfer Agreement (Breast cancer Detection) from EG BioMed * • Licensed Patent and Know - How: methylation analysis technology for application in breast cancer (including U.S. Patent Application No. 17/053,688, Europe 3790984, China Patent No. 201980031343.5,Taiwan Patent No. I721414 and other Patent in Asian countries) and the relevant know - how, and agrees to have the aforesaid patent(s) and relevant know - how • Scope of License: use of the Licensed Patent and Know - How in the Licensed Territory(American, European and Asian) for manufacturing, offering for sale, selling, using, or importing the Product for the aforementioned purposes. High - fat diets contribute to increasing incidence • Method of License: E xclusive license • Authorization tim e : 20 years and will be automatically renewed for another five years or until the expiration of the Licensed Patent » License Fees and Royalties from selling the Product: A royalty of 20% of the " Gross Sales" of the Product on a quarterly basis. *YD Biopharma and EG BioMed have reached an agreement in principle on the above terms and are currently working on definitive documentation. 26 YD BIOPHARMA

YD BIOPHARMA LIMITED THANK YOU