アメリカ合衆国
証券取引委員会
ワシントンD.C.,20549
形式
OR
本財政年度末まで
OR
__________ から __________ への移行期間について ____________
OR
この幽霊会社が報告した事件の日付が必要です
依頼書類番号:
( 登録者の正名 ) 憲章)
適用されない
(登録者氏名英文訳)
(法団または組織の司法管轄権)
電話番号 : 85 2 2155 0 8 2 3
( 主 要 執行 役 所の 住 所 )
最高経営責任者
電話:
(Name、電話、電子メールおよび / またはファクシミルの番号 会社の連絡先及び住所 )
登録または登録予定の証券 同法第 12 条 ( b ) 項の規定
| 各 クラスの タイトル | 取引 シン ボル | 登録された各取引所の名称 | ||
登録または登録予定の証券 同法第 12 条 ( g ) 項に規定する。
なし
(クラス名)
報告義務のある証券 同法第 15 条 ( d ) に基づき、
なし
(クラス名)
各株の発行済株式数を表示します 年次報告書の対象となる期間の終了時点における発行者の資本または普通株式の種類
2024 年 6 月 30 日現在、発行者は
登録者が正しいかどうかをチェックマークで示す
証券法第405条に規定されている経験豊富な発行者。はい。☐
この報告書が年次または移行報告書である場合、
証券取引法第 13 条または第 15 条 ( d ) に基づく報告書の提出が義務付けられていない場合はチェックマークで示します。
1934 年の。はい
見積統計データと予想スケジュール
第四項です。
未解決従業員意見
| 五番目です。 | 経営と財務回顧と展望 | 第六項です。 | 取締役 · 上級管理職 · 従業員 |
| 大株主および関係者取引 | 第八項です。 |
第九項です。
| † | 第10項。 |
情報を付加する
市場リスクの定量的·定性的開示について
株式証券を除くその他の証券説明
パート II
| 14項です。 所有者を担保する権利と収益の使用を実質的に改正する | 第十五項。 |
| * | 制御とプログラム |
プロジェクト16 A。
プロジェクト16 B。
道徳的準則
プロジェクト16 Cです。
| チーフ会計士費用とサービス | ii | |
| プロジェクト16 Dです。 | ||
| 免除監査委員会は上場基準を遵守する | プロジェクト16 E。 | 1 |
| 発行者および関連購入者が株式証券を購入する | プロジェクト16 Fです。 | 1 |
| 登録者の認証会計士を変更する | プロジェクト16 Gです。 | 1 |
| 会社の管理 | 16 H項です。 | 31 |
| 炭鉱安全情報開示 | 16 I 号。 | 55 |
| 検査妨害に関する外国司法管区の開示 | 16 J 。 | 56 |
| INSIDER | 貿易政策S | 67 |
| ITEM 1.6万。 | ネットワーク·セキュリティ | 77 |
| 第三部 | 17項です。 | 80 |
| 財務諸表 | 第十八項。 | 80 |
| 財務諸表 | 「 Regencell Limited 」は、 Regencell Limited を指します。 Regencell は、香港の法律に基づいて組織された香港有限責任会社であり、 Regencell Bioscience Holdings Limited の完全子会社です。 | 81 |
| ● | 「株式」、「株式」または「普通株式」は、 Regencell Bioscience Holdings Limited の普通株式を、 1 株当たり 0.00001 ドルの額面価値とします。 | 89 |
| ● | 「 TCM 」は、中国伝統医学を意味する。 | 89 |
| ● | ||
| 「 TCm プラクティショナー」または「当社の TCm プラクティショナー」は、当社の戦略的 TCm リサーチパートナー、最高経営責任者 ( 「 CEO 」 ) 兼取締役の父である Sik—Kee Au 氏を指します。 | ● | 90 |
| 「 US $」、「 $」または「 US ドル」は、米国の法定通貨を指します。 | ● | 90 |
| 「当社」、「当社」、「 RGC 」、「当社」、「当社」または「グループ」は、 Regencell Bioscience Holdings およびその関連会社の 1 つ以上を指します。 | 普通株式の上場について 2021 年 7 月 16 日に Nasdaq Capital Market に「 RGC 」の記号で上場。 | 90 |
| 第1部 | 項目 1 。取締役、上級管理職の身元 アドバイザーズ | 92 |
| 適用されません。 | 項目2.見積統計データと予想スケジュール | 92 |
| 適用されません。 | プロジェクト3.重要な情報 | 92 |
| 保留されている | キャピタライズ 負債 | 93 |
| ● | 単独でも他人と協力しても、私たちの標準化された液体漢方薬の候補調合の商業販売を発売した | 93 |
| ● | 資本市場やその他の面で十分な資金を集めて、私たちの業務計画を達成すること | 93 |
| ● | 私たちの既存および/または将来調達された資金を利用して、私たちの業務戦略を効率的に実行します | 93 |
| もし成功できなければ 上記のいずれかを実行すると、私たちの業務は失敗する可能性があり、あなたの投資は不利な影響を受けるだろう。 | まだ漢方薬から収入を得ていません 処方候補または任意の規制承認を申請し、流通能力または経験またはいかなる許可特許も持たない、または 未解決の特許出願は、絶対に利益を上げないかもしれない。 | 93 |
| 私たちの収益性は創造力にかかっています 収入を得る。本年度報告の日まで、私たちはまだ何の規制承認も申請していません。私たちはすでに付与されているか、あるいは申請している特許を持っていません。 アプリケーションは流通能力や経験がなく、私たちは開発段階から何の収入も得ていません 私たちはいつ、あるいはそのような収入が発生するのか分からない。大きな収入は発生しないと予想されています 私たちの標準化漢方薬調合が市場の許可を得るまで、これを基礎とする製品を生産と商業化することができます。 漢方処方。私たちが未来に私たちの漢方薬調合に基づく製品販売から収入を得ることができるかどうかは私たちに大きくかかっています。 多くの分野が含まれていますがこれらに限定されません | ● | 93 |
| 良好な研究成果を得て、一定の進展を得た | ●私たちの漢方薬調合の候補を制定し、監督部門の登録許可を得た | 94 |
| ● | 私たちの候補製品のニーズを正確に識別し | 94 |
| ● | ||
| 消費者はADHDとASD症状の治療に引き続き興味を持っている | ● | 95 |
| もし発売が許可されれば、著者らの液体漢方薬調合療法は市場に受け入れられ、ADHDとASDを治療する実行可能な治療方案とする | ● | 95 |
| 私たちが参加する可能性のある任意の協力、許可、または他の手配で有利な条件を交渉する; | ● | 95 |
| 人材を募集して引き留める。 | 97 | |
i
私たちの運営資金源は限られている 多くの追加融資が必要になるだろう
必要な運営資金 私たちの業務計画を実施するためには、私たちの株式、債務、債務リンク証券を発行することで、 そして/または株式に関連した証券、そして未来に私たちが作った収入。十分な収入がある保証はありません 私たちの業務を維持するために、あるいは現在の経済環境下で株式/債務融資を得ることができるだろう。もし私たちがいなければ 十分な運営資金あるいは追加資金を調達して、私たちは完成を延期したり、現在の範囲を大幅に縮小したりするかもしれません。 ビジネス計画;私たちの研究開発を延期する;新しい人員の募集を延期する;あるいはいくつかの恐ろしい財務状況の下で 私たちの業務を大幅に削減または停止する。
十分なものを得ることはできません 追加的な融資は、私たちが業務計画を実施する能力に実質的な悪影響を及ぼすので、必要かもしれません。 アメリカは私たちの行動を大幅に削減するか、または止めるかもしれない。私たちが追加融資を達成する能力は以下の要素にかかっている その他、任意の提案上場時の資本市場状況、市場のわが社に対する受け入れ程度、及び可能性 私たちのビジネスモデルと見積条項の成功。このような追加資本を得ることができるという保証はありません 好ましい条項で、または資産売却、株式または債務融資、または両方の任意の組み合わせを全く介さない。また保証はできません このような融資のいずれかを考慮すると、獲得すれば、私たちの資本需要を満たし、私たちの運営を支援するのに十分であるだろう。もしそうすれば 満足できる条件で十分な資本、私たちの収入と運営、そして私たちの普通株の価値を得ることができません。 普通株式等価物は実質的な否定的な影響を受け、私たちは運営を停止するかもしれない。
私たちの製品開発、規制承認、製造に関するリスク 商業化しています
| 標準化された漢方薬のレシピ候補です 軽,中,重度ADHD,ASD患者では開発中である。もし私たちが規制部門の承認を得られなかったり、最終的に 私たちの漢方薬調合に基づいて、私たちの標準化された漢方薬調合および/または製品を商業化するか、あるいは実行中に重大な遅延があったら したがって、私たちの業務、財務状況、経営業績、見通しは実質的な悪影響を受けるだろう。 | 現在3つの標準化された 軽、中、重度ADHDとASD患者の候補漢方薬調合は研究と開発中である。候補式には一つもありません 現在規制承認と商業化が行われている。私たちは標準化した 漢方薬の調合候補は私たちが完成した研究開発に依存して、監督部門の許可を得て、成功しました このような処方に基づいた製品を商業化することは、決して起こらないかもしれない。私たちの各候補漢方薬の調合には追加の研究が必要です 開発、香港、計画販売の他の管轄区域の規制承認、製造供給の発展と 私たちが製品販売からどんな収入を得る前に、私たちは大量の生産能力、大量の投資、大量のマーケティング作業が必要だ。成功しているところです 軽度、中度、重度の患者に対して、私たちの3つの標準漢方薬調合候補はいくつかの要素に依存する | |
| ● | 採用に成功し研究学習を完成させ | |
| ● | 関連する監督部門の計画中の研究、未来の研究或いは薬物登録、生産と商業化に対する監督管理の許可を得る | |
| ● | すべての安全研究を成功して、香港と他の管轄区の監督管理許可を得て、私たちの標準化漢方薬調合候補者はこれらの司法管轄区で販売しようとしている |
| ● | 私たちの臨床供給と商業製造候補規格に適合した商業製造能力を開発する | |
| ● | 第三者漢方薬原料サプライヤーあるいはメーカーと手配とメンテナンスを行う |
| ● | ● |
| 私たちは適切な能力と経験を持つ人を募集することができます | ● | |
| 類似療法または他の新しい療法の競争的研究は、利用可能な患者の数およびタイプを減少させる | ● | |
| 研究中の漢方薬製剤の他の既存療法に対する潜在的な優位性および副作用に対する患者の見方は、我々が調査している適応のための新薬候補または療法が承認される可能性があることを含む | ● |
| 患者の同意を得て維持する能力は | ● |
| 患者の自然減員状況 | ● |
私たちの候補漢方薬製剤と類似した治療法の利用可能性が承認された。
ii
登録することができても 私たちの治験には十分な数の患者がいて、患者登録の遅延はコストの増加あるいは時間に影響を与える可能性があります あるいは計画された研究の結果、これはこれらの研究の完成を阻害し、私たちの進歩能力に悪影響を及ぼす可能性がある。 中国の漢方医処方の候補の発展。
私たちの初期の個人化の研究結果は 漢方薬調合は未来の治療効果試験結果を予測できないかもしれない。失敗は研究開発のどんな段階でも起こるかもしれない。
研究の結果 著者らの個性化漢方薬調合の結果は標準化漢方薬調合候補の結果を予測できないかもしれない。漢方医の処方を標準化する 個性化された漢方医調合は積極的な結果を得たが、候補者は期待した安全性と有効性を示すことができないかもしれない。否定的な あるいは不確定な結果、私たちは私たちの漢方医従事者または任意の潜在的な未来の協力者と決定するかもしれません、あるいは監督機関は要求するかもしれません。 アメリカでは追加的な研究が行われていますまた,研究から得られたデータは異なる解釈の影響を受けやすい. 規制機関は私たちのように私たちのデータを有利に解読しないかもしれないが、これは規制部門の承認を延期、制限、または阻止するかもしれない。
本年度報告日までの2回目の治験 香港のADHDとASDが進行中である。しかし、私たちの二回目の治療効果試験が再設計する必要があるかどうかは保証されない。研究する. 研究は、遅延または失敗を含む様々な理由で遅延または中止される可能性がある:
●
適切な患者を募集して研究に参加させ、これらの患者に研究或いは帰って治療後のフォローアップを行わせた
A. ●
B. 治療効果試験過程中に出現したいかなる患者の安全問題を解決する
乳香乳香 (Olibanum)
C. ■ ■
没薬 Moyao (Myrrha)
D. 0
トニフィングハーブ:
1
■ ■
アストラガリ黄旗 (Astragali Radix)-■ ■
地黄 Dihuang (Rehmanniae Radix)
■ ■
| 山芋 Shanzhuyu (Corni Fructus) | 0 |
| Qi 調節、熱と風を除去ハーブ : | ■ ■ |
| 晶傑 ( 五葉草 ) | ■ ■ |
| 防風 | ■ ■ |
| 香草香福 ( 香草根 ) | 0 |
| 解毒のハーブ : | ■ ■ |
| 海藻 Haizao, Algae (Sargassum) | ■ ■ | |
| 蒲公英蒲公英 ( Taraxaci Herba ) | ■ ■ |
黄金黄金 ( Scutellariae Radix )
2
0
血栓除去ハーブ :
| ■ ■ | 三七三七 ( Notoginseng Radix et Rhizoma |
| ■ ■ | 唐桂 ( Angelicae Sinensis Radix ) |
| ■ ■ | 川雄川雄 ( 川雄根 ) | |
| 0 | 消化ハーブ: | |
| ■ ■ | バイジュ (Atractylodis Macrocephalae Rhizoma) | |
| ■ ■ | 以下は年内のキャッシュフローの主要な構成要素です 2024年6月30日まで、2023年6月30日、2022年6月30日までの年度。 | |
| 6月30日までの年度 | 経営活動のための現金純額 |
投資活動提供の現金純額
融資活動が提供する現金純額
為替レートが現金に与える影響
3
現金純変動額
現金、年明け
年末現金
| キャッシュフロー | 経営活動 |
| 2024 年および 2023 年 6 月 30 日期 | 上には 2024年と2023年6月30日までの年度の経営活動で使用されている純現金はそれぞれ400ドル万と496ドル万だった。現金純額 経営活動の主な原因は、オフィスやスタッフの寮を借り、私たちの実行者を雇って、研究することです。 そして開発し、マーケティングに力を入れ、一般と行政活動を強化した。 |
| 2023年6月30日と2022年6月30日までの財政年度 | 上には 2023年と2022年6月30日までの年度,経営活動で使用されている純現金はそれぞれ496万と527万であった。現金純額 経営活動の主な原因は、オフィスやスタッフの寮を借り、私たちの実行者を雇って、研究することです。 そして開発し、マーケティングに力を入れ、一般と行政活動を強化した。 |
| 投資活動 | 2024 年および 2023 年 6 月 30 日期 |
| 6月30日までの年度。 2024年と2023年、投資活動が提供する純現金はそれぞれ524万と6万だった。投資提供の現金純額 2024年6月30日までの年間活動は主に短期投資満期によるものであるが、 再投資します。 | 2023年6月30日と2022年6月30日までの財政年度 |
| 6月30日までの年度。 2023年と2022年、投資活動が提供する純現金はそれぞれ6万と1,080ドル。提供した現金純額 2023年6月30日までの年度の投資活動については、主に短期投資の成熟によるものである。論説. 一方,2022年6月30日までの年間投資活動のための現金純額は,主に配給10ドルによるものである 短期投資は100万ドルです。私たちの新しいオフィス空間を改装して、不動産と設備を購入します | 役員および行政員 |
| 執行役員を以下の表に示します。 本契約の日付における取締役及び取締役並びにその年齢及び職務 | 名前.名前 |
| 年ごろ | ポスト |
| ヤット · ガイ · オウ | 取締役会長兼最高経営責任者 |
| ジェームズ · ワイ · ホン · チュン | 取締役、最高執行責任者、最高戦略責任者 |
| ヤット · プイ · オ | 首席商務官 |
エヴァナ · イー · ワ · フイ
4
独立役員
ポール · J · ニーヴァロムスキ
独立役員
Dr. Wing Yan (William) Lo
| 独立役員 | 補償委員会委員長。 |
| 指名 · コーポレートガバナンス委員会委員長。 | 監査委員会委員長。 |
| 2022 年 1 月 1 日、取締役会 2021 年度株式オプション計画に基づき、取締役 15,585 人のオプションを当社の取締役に付与しました。これらのオプションは行使可能です。 1 株当たり 31.85 ドルで、そのうち 25% は IPO 終了後 4 年間の記念日に付与され、 10 年間有効です。 一度服を着た。 | 以下の表は要約です。 本年次報告書の発行日現在、取締役および役員に付与されたオプションの基本となる普通株式の数 2021 年株式オプション計画に基づく役員およびその他の個人に対して、その後没収またはキャンセルされた賞を除く。 関連する助成日。 |
| 名前.名前 | 普通株 |
| 基礎となる | 卓越 |
5
付与オプション
行権価格
( 普通株式 1 株当たり )3ロット期日
有効期限が満了する
グループとしての執行役員
6
2021年6月
2032 年 7 月から 2035 年 7 月までの様々な日付
| グループとしての取締役 | $9.50 から $31.85 まで |
| 2021 年 6 月 ~ 2022 年 1 月までの様々な日程 | 2032 年 7 月から 2036 年 1 月までの様々な日付 |
| グループとしてのその他の従業員 | 2021年6月 |
| 主要執行機関のある国·地域 | 香港.香港 |
| 外国の個人発行業者 | はい |
| 母国法律で開示が禁止されている | 違います。 |
| 役員総数 | 女性は |
| 男性 | 非バイナリ |
| ありません | 公開 |
性別
7
第1部:性別同意
役員.取締役
第2部:人口統計的背景
| 母国管内に在任人数が足りない個人 | LGBTQ+ |
| 人口統計の背景は明らかにされていない | 従業員 |
| 売却株主 | 該当しない。 |
| 薄めにする | 該当しない。 |
| 債券発行の支出 | 該当しない。 |
| 項目10.補足情報 | 資本金 |
該当しない。
定款の大綱および定款細則を組織する
8
必要な情報 表格20-F 10.b項は、当社の目論見書の“株式説明”の節に記載されていますので、詳細は参照のこと 2021年5月31日に採択された再記述された定款は、当社登録番号2改正案の添付ファイル3.2アーカイブとして保存されています。 本年度報告します。
材料契約
私たちはまだ何も締結していない 通常業務過程において本年度報告に述べた以外の重要な契約。
| 外国為替規制 | ケイマン諸島の法律によると 現在、外国為替規制や影響を含む資本輸出や輸入への制限はない。 私たちの株の非住民所有者に配当、利息、または他の支払いを送金します。 |
| 課税 | 以下に以下の要約を示す 私たちの普通株に投資する大きなケイマン諸島香港連邦所得税の結果は 本年度報告の日から発効する法律とその関連解釈は,これらの法律や解釈が変化する可能性がある。この要約は 私たちの普通株への投資に関するすべての可能な税金の結果は言及されていません。例えば 州、地方、その他の税法。 |
| ケイマン諸島の税金 | ケイマン諸島は現在 利益、収入、収益または付加価値に応じて個人や会社に課税しないし、本質的な税収も存在しない 相続税や相続税です。ケイマン諸島政府はわが社に実質的な影響を与える可能性のある他の税金を徴収していません 本司法管轄権の範囲内で署名された文書又は署名後に署名された文書に適用される印紙税は除く ケイマン諸島です。ケイマン諸島はケイマン諸島が発行した株や譲渡された株のために印紙税を支払う必要はありません 島会社(ケイマン諸島の土地権益を持つ会社を除く)。ケイマン諸島は二重税を徴収しません 私の会社やわが社に支払われた任意のお金を支払う条約に適用される。外国為替規制や通貨制限はありません。 ケイマン諸島です。 |
| 配当金および配当金の支払い 私たちの普通株の資本はケイマン諸島で課税する必要もなく、源泉徴収も必要ありません。 私たちの普通株式の所有者に配当金や資本を支払っても、売却から収益を得ることはありません 私たちの普通株はケイマン諸島の所得税あるいは会社税を払わなければなりません。 | この要約は 1986年に改正された“国内所得法”(以下、“規則”という。)は、本年度報告の日から発効し、米国財務省で発効した。 本年度報告の日までに施行される又は場合によっては提案された条例及び司法及び行政解釈 その日付または前に準備しておきます。これらの当局は変化するかもしれないし、異なる解釈があり、追跡力があるかもしれません。 基礎です。 |
| この議論は触れていない 米国の所持者の個人状況によると、米国の所持者に関連する米国連邦所得税のすべての面がある可能性がある。 特に,本議論では我々の普通株を持つ米国所有者のみを対象とする. 守則“第1221条(一般に、投資のために保有する財産)。この議論は代替案の潜在的な応用にも触れていない 最低税、ある純投資収入に対して徴収される連邦医療保険納付税、あるいはアメリカ連邦所得税の米国保有者に対する結果 特別なルールに制約されたルールは、以下のことを含む | ● |
| 金融機関や金融サービス実体; | ● |
| 証券、商品または通貨のブローカー、トレーダー、またはトレーダー; | ● |
| 時価会計の者を選択する | ● |
免税エンティティ、“個人退職口座”または“Roth IRA”
9
●
政府や機関やその道具
●
| 保険会社 | ● |
| 規制された投資会社 | ● |
| 不動産投資信託基金 | ● |
| 外国人や元アメリカの長期住民もいます | ● |
| 私たちが発行した普通株式の5%以上を投票権または価値に応じて実際にまたは建設的に所有する人 | ● |
| 従業員株式オプションの行使、従業員株式インセンティブ計画または他の補償に関する方法で私たち普通株を獲得した者 | ● |
| アメリカ国外の貿易や業務に関連して私たちの普通株を持っている人 | ● |
| 国境を越えた、推定販売、満期保証、転換、または他の総合取引の一部として私たちの普通株を持っている人;または | ● |
| 機能通貨はドルの人ではない。 | この議論は触れていない 米国連邦非所得税法の任意の態様、例えば贈与法または遺産税法、または州、地方、または非米国税法。またこの点は 米国連邦所得税の目的を考慮せずに組合企業に分類された実体や手配された税収待遇を検討する またはそのようなエンティティによって、または私たちの普通株式を保有する他の伝達エンティティまたは個人を配置する。提携企業(またはその他)であれば アメリカ連邦所得税の目的のために組合企業の実体や手配に分類される)は、私たちの普通株の実益所有者である。 組合企業におけるパートナーのアメリカ連邦所得税待遇は通常パートナーの地位と活動に依存します。 協力関係の一部です本議論では私たちの普通株式とどんなものについても 米国の保有者がそのような株式を売却またはその他の方法で処分することにより受領された(または受領とみなされる)対価格は ドルです。 |
| 私たちは求めていません アメリカ国税局(IRS)の裁決やアメリカ連邦収入に関する弁護士の意見を求めるのではなく ここで説明された税金の結果。国税局は、本明細書で議論された1つまたは複数の態様に同意しない可能性があり、その決定は、 裁判所の支持を得た。また、未来の立法、条例、行政裁決、または裁判所判決を保証することはできない。 今回の討論で発言の正確性に悪影響を与えないだろう。 | 複雑だからです 私たちの普通株式の特定の所有者の税金結果は未検討事項の影響を受ける可能性があるからです。 ここでは、普通株式のすべての所有者に税務顧問に相談することを促します。 州、現地、非アメリカ税法の適用性と効力を含む、私たちの普通株式の所有権と処分 アメリカ連邦税法と適用されるどんな税金条約も。 |
| 受動的対外投資会社 | アメリカではない会社は 米国連邦所得税用途のPFICは、いかなる課税年度においても、ある検査規則を適用した後、以下のいずれかである |
| ● | 総収入の少なくとも75%は受動的収入です |
10
●
同社の資産の少なくとも50%は、1つの課税年度内の当該資産の四半期価値平均値で計算されるのが一般的であり、受動的な収入を生成するために保有する資産に起因することができる。
上記の目的について言えば 計算すると、非米国会社はその割合の資産シェアを持ち、その比例シェアを稼ぐとみなされる。 それは、25%以上の(価値によって計算される)株式を所有する任意の他の会社の収入を直接または間接的に所有する。受動的収入 一般に、配当金、利息、あるレンタル料または特許使用料、外貨または他の投資収益、およびいくつかの他のカテゴリが含まれています。 収入の割合。私たちは各課税年度に私たちが個人投資会社であるかどうかを別途決定しなければなりません。
101.INS*
インライン XBRL インスタンスドキュメント — インスタンスドキュメントは、その XBRL タグがインライン XBRL ドキュメント内に埋め込まれているため、インタラクティブデータファイルには表示されません。
101.Sch*イントラネットXBRL分類拡張アーキテクチャ文書101.カール*インラインXBRL分類拡張計算リンクライブラリ文書101.定義*インラインXBRL分類拡張Linkbase文書を定義する101.実験所*XBRL分類拡張ラベルLinkbase文書を連結する101.前期*インラインXBRL分類拡張プレゼンテーションLinkbaseドキュメント表紙相互データファイル(添付ファイル101に含まれるイントラネットXBRLのフォーマット)
本局に提出します。
11
手紙で提供する。
署名
登録者は証明する フォーム 20—F に提出するためのすべての要件を満たしており、下署名者に署名することを正当に許可した。 年次報告書を提出。
| レジェンセルバイオサイエンスホールディングス | 投稿者: |
| / s / Yat—Gai Au | 名前: |
| ヤット · ガイ · オウ | タイトル: |
最高経営責任者
( 執行役員 )
日付:
2024 年 10 月 25 日
12
レジェンセルバイオサイエンスホールディングス株式会社
連結財務諸表索引
ページ
独立公認会計士事務所レポート(PCAOB ID:
独立公認会計士事務所レポート(PCAOB ID:
財務諸表:
13
2024 年 6 月 30 日および 2023 年 6 月 30 日連結バランスシート
2024 年 6 月 30 日期、 2023 年 6 月 30 日期、 2022 年 6 月 30 日期連結業績計算書
2024 年 6 月 30 日期、 2023 年 6 月 30 日期および 2022 年 6 月 30 日期における株主資本 ( 赤字 ) の連結計算書
14
2024 年 6 月 30 日期、 2023 年および 2022 年期連結キャッシュ · フロー計算書
F—7 から F—8
連結財務諸表付記
F—9 から F—22
財産と設備、純額
長期預金
15
使用権資産、純額
その他資産総額
| 総資産 | 負債と株主権益 | |
| 流動負債 | 発生経費 | |
| その他の債務 — 関連当事者 | レンタル負債を経営しています--流動負債 | |
| 流動負債総額 | 非流動負債 | |
| 営業リース負債 ( 非経常 ) | 負債総額 | |
| 引受金とその他の事項 | 株主権益 | |
| 普通株、$ | パー値、 | |
| 許可された株式と | 2024 年 6 月 30 日現在、 2023 年 6 月 30 日現在発行済株式 | |
| 追加実収資本 | 赤字を累計する | |
| その他の総合損失を累計する | 当社の株主に帰属する株式総額 | |
| 非制御的権益 | 株主権益総額 |
総負債と株主権益
16
付属の注釈は、 連結財務諸表です
株式会社レジェンセルバイオサイエンスホールディングスおよび子会社
統合 営業 · 損失計算書
6 月 30 日を末日とする年度は、
運営費用:
販売とマーケティング一般および管理 ( 株価報酬 $含 ) 百万、ドル
百万ドルとドル
2024 年 6 月 30 日、 2023 年 6 月 30 日、 2022 年 6 月 30 日 ) 。
17
研究開発 ( 株価報酬の逆転を含む )
100 万ドル、株式ベースの報酬
百万ドルとドル
| 2024 年 6 月 30 日、 2023 年 6 月 30 日、 2022 年 6 月 30 日 ) 。 | 総運営費 |
| 運営損失 | その他の収入、純額 | |
| 所得税前損失 | 所得税支給 |
純損失
以下に起因する損失はありません。
会社の株主
18
非制御的権益
その他総合損失
外貨換算調整
総合損失
以下に起因する包括的な損失なし :
19
当社の株主
非制御的権益
普通株式の加重平均数
基本的希釈の
1株当たり損失
基本的希釈の
付属の注釈は、 連結財務諸表です
株式会社レジェンセルバイオサイエンスホールディングスおよび子会社
統合 株主自己資本 ( 赤字 ) の変動に関する明細書
20
普通株
その他の内容
| 支払済み | 積算 | |
| その他 | 総合的 |
積算
ノン
制御する
株価
額面 . 額面
21
資本
損
赤字.赤字
利子
総額
BALANCE 、 2021 年 6 月 30 日
株式公開時および超過配分時における株式発行、純
22
株式承認証を発行する
転換社債の転換による株式発行
株式ベース報酬
子会社の非支配権益による出資
| 株式証の行使 | 純損失 |
| BALANCE 、 2022 年 6 月 30 日 | 株式ベース報酬 |
| 子会社の非支配権益からの出資 | 純損失 |
| 外貨換算調整 | バランス、 2023 年 6 月 30 日 |
| 株式ベース報酬 | 子会社の非支配権益からの資本出資 ( 純 ) |
| 純損失 | 外貨換算調整 |
| バランス、 2024 年 6 月 30 日 | 付属の注釈は、 連結財務諸表です |
| 株式会社レジェンセルバイオサイエンスホールディングスおよび子会社 | 統合 キャッシュフロー計算書 |
| 6 月 30 日を末日とする年度は、 | 経営活動のキャッシュフロー: |
純損失
純損失と経営活動で使用される現金純額の調整:
減価償却
23
使用権資産の償却
株式ベースの報酬
利 子 収入
財産と設備処分損失
経営性資産と負債変動
| 前払金その他売掛金 | 長期預金 |
| 発生経費 | リース負債を経営する |
| その他の債務 — 関連当事者 | 経営活動のための現金純額 |
投資活動によるキャッシュフロー:
財産と設備を購入する
財産 · 設備の処分を進めること
短期投資の成熟期から進める
短期投資の配置.
24
投資活動提供の現金純額
資金調達活動のキャッシュフロー:
株主貸付金の返済
株式公開の収益 ( ネット )
子会社の非支配権益からの資本出資 ( 純 )
融資活動が提供する現金純額
25
為替レートが現金に与える影響
現金で両替しない
現金、年初
年末のキャッシュ
株式会社レジェンセルバイオサイエンスホールディングスおよび子会社
統合 キャッシュ · フロー決算 ( 続き )
26
キャッシュフロー情報の追加:
所得税の現金を納める
| 利子を支払う現金 | 非現金融資活動の追加開示: |
| 関連債権の可換証券の普通株式への転換 | 令状の発行 |
| 運営部門です。会社のCODM管理 資源を分配するために、会社は総合ベースで運営している。会社所有の長期資産 すべて香港で行われます。 | 外貨換算と取引 |
| 会社の報告金種はドルだ ドル(“$”)。当社は香港でローカル通貨香港ドル(“香港ドル”)で業務を経営している。 その機能通貨として。総合貸借対照表勘定、経営報告書勘定、権益勘定はすべて換算されました。 香港金融管理局(“金管局”)が発表した為替レートで計算する。会社は為替リスクを考慮している 香港ドルの値での取引はドルに対して重要ではありません。香港ドルはドルにリンクしているからです。 | 貨幣資産と負債は 本位貨幣以外の通貨は残高に存在する為替レートで原価ビットコインを換算する 図面日付。年内に本位貨幣以外の貨幣種で行った取引換算を本位貨幣とする 取引日の適用為替レート。取引損益は“他の収入” Net“です外貨建ての資産と負債を貸借対照表の日の為替レートで換算する。権益. 勘定は歴史的為替レートで換算し,収入·費用·損益は年平均為替レートで換算する この一年です。翻訳調整報告書は累積翻訳調整であり、他の包括的なものとして表示されている 合併株主損失と全面赤字変動表における損失。 |
| 現金 | 現金は銀行に預けた当座預金です。 またはその他は引き出しや使用制限を受けず、元の存続期間は3ヶ月または もっと少なくて、既知の数量の現金に両替しやすいです。2024年6月30日と2023年6月30日までのすべての現金残高は財務年度に維持されている 香港預金保障委員会が保証を受けて、最高保険額は香港ドルです |
| ( 約 $ | ) 銀行が倒産した時、計画メンバーのすべての預金者がいた。 |
| 短期投資 | 短期投資代表証明書 購入日、満期日が1年未満の銀行預金や定期預金のこと。 |
| 財産と設備、純額 | 財産と設備をコストを差し引いた価格で列記する 減価償却と減価償却損失(ある場合) |
| 減価償却は直線法で見積もりの有用な価値を計算する 資産の生命。予想される寿命は以下のとおりである | 有益な生活 |
| 賃借権改善 | 残りのレンタル期間のうちの短いものまたは |
| 使用可能寿命を見積もる | オフィス家具、設備、その他 |
| 年間 | 機動車 |
| オフィス家具 · 機器等 | 機動車 |
| 総額 | 減算:減価償却累計 |
| 総額 | 6 月末の減価償却費 30 、 2024 、 2023 、 2022 は約 $ |
と $
27
それぞれ。
2024 年 6 月 30 日期、 2023 年 6 月 30 日期、 2023 年 6 月 30 日期 2022 年、資産 · 設備の処分損失は約
ゼロ
そして
ゼロ
それぞれ。
注 4 : 経費発生未払い経費は給与 · 福祉です 支払金、専門手数料、ユーティリティ、その他の営業費用の支払金。.”
28
注 5 関連当事者取引
その他の債務 — 関連当事者
2022 年 6 月 30 日期は、当社は エース · ユナイテッド · インターナショナル · リミテッドとオフィスの賃貸契約を締結しており、設立者の完全所有会社です。 CEO 。同社は毎月の家賃を支払っています。
契約の条件は毎年更新されますレンタル契約書 2021 年 12 月に終了。家賃料は発生したまま支出されます。2024 年 6 月 30 日、 2023 年、 2022 年の間、 家賃は
ゼロ
6 月 30 日の時点で 2024 年、 2023 年。
29
注 6 — 税金
所得税
所得税規定は、以下の構成要素で構成されています。
6 月 30 日を末日とする年度は
現在:
30
香港.香港
A. 延期:
香港.香港
所得税引当総額
ケイマン諸島および英領ヴァージン諸島 ( BVI )
ケイマン諸島の現行法の下で BVI 、当社およびその子会社は、所得またはキャピタルゲインに課税されません。さらに、配当金の支払に際して ケイマン諸島や BVI の源泉徴収税は課されません
香港.香港
香港で法人化された事業体は対象となる 香港の利益税に
2024 年 6 月 30 日、 2023 年および 2022 年 6 月 30 日を末日とする年度の評価可能利益の推定値の% 。
以下の表は法定税率の調整 下記の期間における会社の実効税率に :
6 月 30 日を末日とする年度は
31
法定税率で計算される税制優遇措置
推定免税額
| 実際の税率 | 次の表は重要なものを示している 繰延税金資産総額の構成要素: |
| 6月30日まで | 繰延税金資産: |
| 営業純損失繰り越し | 株式ベースの給与費用 |
| 減算:推定免税額 | 繰延税項目純資産 |
次の表に中の変更を示します 推定免税額:
この年度までに
6 月 30 日
年初残高
追加する
減算:反転
32
年末の残額
繰延税金資産の最終現金化 このような一時的な差額が控除可能な期間の未来の課税所得額になることに依存する。管理する. この評価を行う際には,累積収益と予想される将来の課税所得額を考慮すべきである。基本的にすべてを回復しました 会社の繰延税金資産は将来の収入の発生に依存し、輸出課税の一時的な差は含まれていない。 過去の経営実績と将来の課税収入の予測によると、経営陣は 2024年6月30日と2023年6月30日までの年度の繰延税金資産。2024年6月30日と2023年6月30日までの税損繰越額は約 $
B. 百万ドルとドル
百万は,それぞれ無期限に繰り越すことができる.
会社は不確定な税務状況を評価しています (利息および罰金の潜在的適用を含む)技術的メリットに基づいて、未確認の利益を測定する 税務頭寸に関連しています。2024年6月30日と2023年6月30日まで、会社には重大な未確認の不確定税はありません。 位置について。当社は6月30日まで、潜在的な所得税の過払いに関する利息や罰金を招いていません。 2024年と2023年。当社の主な税務管区は香港です。2018年から2024年までの納税年度はまだ審査が必要です 香港税務局(“香港税務局”)が提供する。その会社も大きな成長や 2024年6月30日から今後12カ月以内に確認されていない税収割引が減少した。
| 注記 | 7-株主権益(損失) | |
| 6 月 | リスクフリー金利 | |
| オプションの期待寿命 | 年間 |
| 予想ボラティリティ | 期待配当収益率 | |
| 公正価値 | 二項オプション価格モデル | |
| オプション | 授与された | |
| 1 月 | オプション付与日における普通株式の公正価値 |
33
リスクフリー金利
1%から3%
オプション寿命
年間
予想ボラティリティ
1%から3%
34
期待配当収益率
実行価格
予想される早期運動複数
6 月期における株式オプションの活動概要 30 、 2024 、 2023 は次のように示されます。
数量
35
シェア
オプション
重みをつける 平均 運動
価格
重みをつける
平均
付与日の公正価値重みをつける 平均
36
残り 契約 期日骨材 固有 価値年.年2022 年 7 月 1 日現在授与する期限切れ、没収、またはキャンセル2023 年 6 月 30 日現在未払い
授与する
期限切れ、没収、またはキャンセル
2024 年 6 月 30 日現在未払い
2024 年 6 月 30 日現在行使可能2024年6月30日までの年間で、当社は 確認された株式ベースの報酬支出は約$ (2023: $3)は、付与された株式オプションに適用される。
37
株式承認証を公開発売する
公共活動に関連して大衆活動の終了時に 当社はそれぞれ2021年7月20日および2021年8月19日に発売および超過配給を行い、株式2.5%相当の引受権証を発行する 公開発売では,合わせて57,500単位および8,125単位を配給エージェントに発売した(以下,“公開発売”と呼ぶ). 手令“)。株式承認証の有効期間は5年であり、取引終了後180日以内に行使してはならない。 公開発行は、1株10.45ドル相当の価格で行使できる。
経営陣はこれらの株式承認証が定義に合っていると確定した しかし,ASC 815-40でのデリバティブについては,それらは範囲例外であり,その範囲例外は,発行された契約はすべてa)インデックスであることを規定している. (B)株主権益に帰属するものはデリバティブとはみなされない.株式証明書はその公正価値に従って入金される. 付与された日に株主不足の一部とする。
2021年7月20日の引受権証は$
百万ドルです。公正価値はブラック·スコアーズ定価モデルを用いて推定され、その式は以下のようになる。 加重平均仮定:基礎株式の時価は#ドル
38
無リスク金利
予想期限は
株式証明書金額は$
リースコストを経営する
研究開発費
短期賃貸コスト
一般と行政費用
リースに関するバランスシート情報は 以下のもの :
39
分類する
六月三十日
六月三十日
資産
オペレーティングリース — ROU 資産
使用権資産
負債.負債
リース負債を経営する
流通部分
リース負債を経営する
40
非電流部分
リース総負債
加重平均残存期間 ( 年 )
賃貸借契約を経営する
加重平均割引率賃貸借契約を経営する以下の表は、会社の 今後の最低賃貸料支払額 :
六月三十日
6 月 30 日までの年度は、
最低賃貸支払総額
41
差し引く:推定利息
合計:
付記9--支払引受及び又は事項
緊急事態
当社は、随時、 特定の法的手続、請求および通常の業務の過程で生じる紛争に。これらの法的結果にもかかわらず 当社は、これらの行為が、総じて、重大な悪影響を及ぼすとは考えません。 財務状況、営業結果または流動性です
注記
10. その後の出来事その後のイベント · 取引の評価 10 月 25 日の連結財務諸表発行日までの貸借対照表期日以降に発生した 2024.審査に基づき、当社は、その後の調整または開示を必要とする事象を特定していません。 連結財務諸表。誤り会計年度残りリース期間または推定耐用年数の短いデイ: ビジネスコンタクトメンバーrgc: AuditorInformationMember米国-公認会計基準:関連側メンバー米国-公認会計基準:関連側メンバー
アメリカ-公認会計基準:一般と行政費用メンバー
アメリカ-公認会計基準:一般と行政費用メンバー
42
アメリカ-公認会計基準:一般と行政費用メンバー
米国-公認会計基準:研究·開発費メンバー
米国-公認会計基準:研究·開発費メンバー
米国-公認会計基準:研究·開発費メンバー
アメリカ-アメリカ公認会計基準:普通株式メンバー
US-GAAP:AdditionalPaidInCapitalMembers
アメリカ公認会計原則:他の総合収入メンバーを累計
アメリカ-公認会計基準:前払いメンバーを保留
アメリカ公認会計基準:非制御的利益メンバー3アメリカ-アメリカ公認会計基準:普通株式メンバー
43
US-GAAP:AdditionalPaidInCapitalMembers
アメリカ公認会計原則:他の総合収入メンバーを累計
アメリカ-公認会計基準:前払いメンバーを保留
アメリカ公認会計基準:非制御的利益メンバー
アメリカ-アメリカ公認会計基準:普通株式メンバー
US-GAAP:AdditionalPaidInCapitalMembers
アメリカ公認会計原則:他の総合収入メンバーを累計
アメリカ-公認会計基準:前払いメンバーを保留
アメリカ公認会計基準:非制御的利益メンバー
アメリカ-アメリカ公認会計基準:普通株式メンバー
US-GAAP:AdditionalPaidInCapitalMembers
アメリカ公認会計原則:他の総合収入メンバーを累計アメリカ-公認会計基準:前払いメンバーを保留アメリカ公認会計基準:非制御的利益メンバー
44
アメリカ-アメリカ公認会計基準:普通株式メンバー
US-GAAP:AdditionalPaidInCapitalMembers
アメリカ公認会計原則:他の総合収入メンバーを累計アメリカ-公認会計基準:前払いメンバーを保留アメリカ公認会計基準:非制御的利益メンバー
アメリカ-アメリカ公認会計基準:普通株式メンバー
US-GAAP:AdditionalPaidInCapitalMembers
| アメリカ公認会計原則:他の総合収入メンバーを累計 | アメリカ-公認会計基準:前払いメンバーを保留 |
| アメリカ公認会計基準:非制御的利益メンバー | アメリカ-アメリカ公認会計基準:普通株式メンバー |
| US-GAAP:AdditionalPaidInCapitalMembers | アメリカ公認会計原則:他の総合収入メンバーを累計 |
| アメリカ-公認会計基準:前払いメンバーを保留 | アメリカ公認会計基準:非制御的利益メンバー |
| 米国-GAAP:IPOメンバー | 米国-GAAP:IPOメンバー |
| 米国-GAAP:IPOメンバー | アメリカ-アメリカ公認会計基準:普通株式メンバー |
| アメリカ公認会計基準:超過割当オプションメンバー | rgc: MrYatGaiMember |
| アメリカ-アメリカ公認会計基準:普通株式メンバー | rgc : MrYatGaiMember |
| rgc : MrYatGaiMember | rgc: RegencellBioscienceAsiaLimited メンバー |
45
| rgc: RegencellBioscienceLimited メンバー | rgc: RegencellLimitedMember |
| rgc: RegencellBioscienceAsiaLimited メンバー | rgc: RegencellBioscienceNorthAmericaLimited メンバー |
| SRT:最小メンバ数 | SRT:最大メンバ数 |
| アメリカ公認会計基準:レンタルとレンタル改善メンバー | アメリカ公認会計基準:レンタルとレンタル改善メンバー |
| アメリカ-GAAP:OfficeEquipmentMembers | アメリカ-GAAP:OfficeEquipmentMembers |
| アメリカ-GAAP:車両メンバー | アメリカ-GAAP:車両メンバー |
| rgc: MrChungMember | rgc: MrAuMember |
| rgc: MrChungMember | rgc: MrAuMember |
| rgc: RegenerationCompanyLimited メンバー | rgc: RegenerationCompanyLimited メンバー |
| rgc: RegencellBioscienceAsiaSdnBhd メンバー | rgc: RegencellBioscienceAsiaSdnBhd メンバー |
| rgc: RegencellBioscienceHoldingsLimited メンバー | rgc: RegencellBioscienceHoldingsLimited メンバー |
| アメリカ-アメリカ公認会計基準:普通株式メンバー | アメリカ公認会計基準:超過割当オプションメンバー |
| rgc: ShareBasedPaymentArrangement ベストメンバー | rgc: ShareBasedPaymentArrangement ベストメンバー |
| rgc: BlackScholes オプション価格モデルメンバー | rgc: BlackScholes オプション価格モデルメンバー |
| rgc: BinomialOption 価格モデルメンバー | rgc: OptionsMember |
| SRT:最小メンバ数 | rgc: OptionsMember |
SRT:最大メンバ数
rgc: OptionsMember
rgc: OptionsMember
xbrli: 株式
46
iso4217: USD
iso4217: USD
xbrli: 株式
xbrli: 純粋
ISO 4217:香港ドル
Each enrolled patient in our first research study stopped all his or her existing medical treatment for ADHD and/or ASD and was treated with a personalized TCM formulae for a period up to three months. Patient’s symptoms were assessed through parent interviews and using the globally accepted assessment tools, which were provided below. The parent’s qualitative and quantitative assessment scores on the patient’s behavior were compared before, during and after three months of treatment. The assessment tools used were the questionnaires filled in by the parents, which included (i) Sik-Kee Au TCM Brain Theory® for ADHD/ASD Assessment (SKATBT-A3); (ii) Autism Treatment Evaluation Checklist (ATEC); (iii) Gilliam Autism Rating Scale (GARS); (iv) Vanderbilt ADHD Diagnostic Parent Rating Scale (VADRS); and (v) Swanson, Nolan, and Pelham (SNAP)-IV 26-item Parent Rating Scale (SNAP-IV-26), which covered the first 26 items of the VADRS, for further analysis. The individual assessment method is explained in detail below. The assessment tools used in our research are globally recognized and accepted methods of scoring severity levels of ADHD and ASD patients.
The first research study was only the first phase of our research studies. Our uncontrolled first research study was designed with a small sample size to allow us to collect preliminary data, monitor the treatment progress, observe the effect of the treatment and note any adverse side effects in a cost-effective and systematic way. It has helped us better prepare for our second efficacy trial which started in August 2021. We intend to conduct further efficacy trials with a larger sample size using the three standardized TCM candidates.
47
Although most of these are globally recognized assessment tools for the assessment of ADHD and ASD, the outcome of our efficacy trial may be subject to some biases of parents and caregivers of patients because we relied on the data provided by them, such biases may come from parental expectations, social desirability and recall bias. If our understanding and use of these assessment tools is flawed, or if the parents and caregivers of enrolled ADHD and ASD patients fail to observe and record accurately, then we will not only fail to realize any benefits from using these assessment tools, but may also be led to invest time and financial resources inefficiently in attempting to develop inappropriate TCM formulae candidates.
Sik-Kee Au TCM Brain Theory® for ADHD/ASD Assessment (SKATBT-A3)
SKATBT-A3 is a 48-item questionnaire developed by the research and development team of Regencell, designed to assess patients’ conditions that are commonly observed by the TCM Practitioner. Items assessed are indications of patients’ overall body and neurological conditions based on the TCM Practitioner’s brain theory and his over 30 years of experience in treating ADHD and ASD patients. SKATBT-A3 has a total score ranging from 0 to 122, the higher the score, the more problematic the symptoms. The severity of the patient’s condition is categorized into three groups of equal total score range: mild (total score 0-40), moderate (total score 41-80) and severe (total score ≥ 81).
Autism Treatment Evaluation Checklist (ATEC)
ATEC is specially designed to measure changes in autistic severity, making it useful in monitoring behaviors over time as well as tracking the efficacy of a treatment. ATEC comprises of four subscales: (1) Speech/Language/ Communication, (2) Sociability, (3) Sensory/Cognitive Awareness and (4) Health/Physical/Behavior. ATEC is a caregiver-administered questionnaire designed to measure changes in the severity of autism in response to treatment. The scores from each subscale are combined in order to calculate a Total Score. A lower score indicates a lower severity of autism symptoms and a higher score correlates with more severe symptoms of autism.
Gilliam Autism Rating Scale (GARS)
GARS is one of the most widely used instruments for the assessment of ASD in the world. It is a 42-item norm-referenced screening instrument used for the assessment of individuals ages 3-22 who have severe behavioral problems that may be indicative of autism. It gathers information about specific characteristics typically noted in children with ASD in three areas: stereotyped behaviors, communication and social interaction. It also contains a Developmental Disturbances section. As the questions included in the developmental disturbances section of GARS refer to the condition of the patient during the patient’s first 36 months of age, the total score is calculated by adding the sum of the raw scores of the three subscales of stereotyped behaviors, communications and social interaction, but excluding the scores of developmental disturbances. A lower score indicates a lower severity of autism symptoms and a higher score correlates with more severe symptoms of autism.
Vanderbilt ADHD Diagnostic Parent Rating Scale (VADRS)
VADRS is a psychological assessment tool for parents of children aged 6 to 12 designed to measure the severity of ADHD symptoms. Developed by Mark Wolraich at the Oklahoma Health Sciences Center, this rating scale also includes items related to other disorders which are frequently comorbid with ADHD. Similar to ATEC and GARS, a lower score indicates a lower severity of ADHD symptoms and a higher score correlates with more severe symptoms of ADHD.
Swanson, Nolan, and Pelham (SNAP)-IV 26-item Parent Rating Scale (SNAP-IV-26)
SNAP-IV-26, which is an abbreviated version of the SNAP Questionnaire (Swanson, 1992; Swanson et al., 1983) and the same as the first 26 items of the VADRS, was also used. Items from the DSM-IV criteria for ADHD are included in the two subsets of symptoms: Inattentive (items 1 – 9) (score range from 0 – 27) and Hyperactivity/Impulsivity (items 10 – 18) (score range from 0 – 27). Also, items from the DSM-IV criteria for oppositional defiant disorder (ODD) are included (items 19 – 26) (score range from 0 – 24) because ODD is often present in children with ADHD. The lower the SNAP-IV-26 score, the fewer the problems. The scores provide an interpretation for severe, moderate, mild and no clinically significant symptoms.
48
Our Second Efficacy Trial in ADHD and ASD
We commenced an uncontrolled second efficacy trial in August 2021, to evaluate the effects of our standardized TCM formulae by arranging them to be treated by the TCM Practitioner with the use of our standardized TCM formulae for up to three months. Requirements for participants were the same as those in our first research study. A total of twenty-eight (28) patients in Hong Kong, who were clinically diagnosed with ADHD and/or ASD by their healthcare professionals at different levels of severity, voluntarily consented through their parents or guardians to participate in our second efficacy trial. Seven (7) of them started in August 2021 as the first group, fourteen (14) started in July 2022 as the second group and seven (7) started in October 2022 as the third group. The enrolled patients’ ages ranged from five to thirteen years old. We intend to conduct further efficacy trials going forward.
Our second efficacy trial on the three groups of patients showed similar improvements in patients’ symptoms. Within three months of receiving treatment using our standardized TCM formulae, our research showed that patients had attained better speech, communication, sociability, cognition and behavioral abilities. Their overall health, sleep quality and appetite had also improved. As part of the improvement process, patients also experienced better bowel movement, sweating and temporary fatigue, which were expected by our TCM Practitioner. A summary of the interim report can be found on our website at https://www.regencellbioscience.com under patient case studies tab.
Assessment Methodology
Each enrolled patient in our second efficacy trial stopped all his or her existing medical treatment for ADHD and/or ASD and was treated with a standardized TCM formulae for a period of three months. Patient’s symptoms were assessed through parent interviews and using the globally accepted assessment tools, the Autism Treatment Evaluation Checklist (ATEC) and the Vanderbilt ADHD Diagnostic Parent Rating Scale (VADRS) questionnaire. We also adopted the Sik-Kee Au TCM Brain Theory® for ADHD/ASD Assessment (SKATBT-A3), which is a 48-item questionnaire developed by the R&D team of Regencell Bioscience, designed to assess patients’ conditions that are commonly observed by the TCM Practitioner. Items assessed are indications of patients’ overall body and neurological conditions based on the TCM Practitioner’s brain theory and his over 30 years of experience in treating ADHD and ASD patients. The Autism Treatment Evaluation Checklist (ATEC) and the Vanderbilt ADHD Diagnostic Parent Rating Scale (VADRS) questionnaire again as our assessment tools in our second efficacy trial. In addition, we had used additional assessment tools, which were also parents’ filled questionnaires, to obtain different measurements of patients’ condition for evaluation. The parent’s qualitative and quantitative assessment scores on the patient’s behavior were compared before, during and after three months of treatment.
Our Leases
We currently maintain offices at Office A & B on 11th Floor, First Commercial Building, 33-35 Leighton Road, Hong Kong. We entered a five-year fixed term lease agreement for the office space on July 15, 2019. The lease expires on July 14, 2024.
We have a separate office rental agreement with Ace United International Limited, which is a company wholly-owned by our founder and CEO. The monthly rent is $4,103 for the premises located at 21st Floor, EIB Tower, 4-6 Morrison Hill Road, Wan Chai, Hong Kong. The agreement is renewable on an annual basis. The rental payment is expensed as incurred. The agreement was terminated in December 2021.
We entered into a three-year lease agreement for an office space at 9th floor, Chinachem Leighton Plaza, 29 Leighton, Causeway Bay, Hong Kong, on August 9, 2021.
During the years ended June 30, 2024 and 2023, we entered into agreements to lease usage of staff quarters with third parties. Each of the leases was for a two-year term.
Regulation Related to our Business Operation in Hong Kong
The Legislative Council of Hong Kong, Chinese Medicines Council, Chinese Medicines Board and Chinese Medicines Regulatory Office of Department of Health of Hong Kong are independent and separate entities from China. Whilst Hong Kong is part of the PRC, Hong Kong and the PRC are regarded as two separate jurisdictions in law and markets. Under the “One Country, Two Systems”, Hong Kong retains its own systems and way of life after the handover of sovereignty to the PRC in 1997. The Hong Kong Basic Law, being Hong Kong’s constitutional document, gives legal effect to the “One Country, Two Systems” policy.
49
Pursuant to the Basic Law, the laws previously in force in Hong Kong, that is, the common law, rules of equity, ordinances, subordinate legislation and customary law shall be maintained, except for any that contravene the Basic Law, and subject to any amendment by the Legislative Council of Hong Kong. The socialist system and policies shall not be practiced in Hong Kong, and the previous capitalist system and way of life shall remain unchanged for 50 years. Further, Hong Kong residents and other persons in Hong Kong shall have the obligation to abide by the laws in force in Hong Kong.
As we operate in Hong Kong, we have no assets or operation in PRC, nor have plan to commence operation in PRC, we do not believe that PRC laws or regulations regarding the TCM industry apply to us. Hence, within Hong Kong’s legal system, the laws and regulations of the PRC do not apply.
Hong Kong Chinese Medicine Ordinance (HKCMO)
The HKCMO provides licensing requirements for the sale and distribution of our TCM formulae candidates, registration and licensing requirements of our Chinese healthcare products, and future operations of our Chinese medicine clinics in Hong Kong. The HKCMO was passed by the Legislative Council of Hong Kong on July 14, 1999. The HKCMO is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the HKCMO in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Food and Health Bureau, the Department of Health and etc., as well as the Chinese Medicine Council of Hong Kong, have the implementation rights of the HKCMO. The Chinese Medicine Council of Hong Kong is the main regulator of the HKCMO.
Registration of TCM products and Chinese Healthcare Products
Some of our TCM products and Chinese healthcare products are classified as proprietary Chinese medicine (pCm), defined below, and others are classified as non-pCm. The key differences between pCm and non-pCm lie on their ingredients, dosage forms and intended use. Under section 2 of the HKCMO, pCm is defined as any proprietary product, which is formulated in a finished dose form and is known or claimed to be used for the diagnosis, treatment, prevention or alleviation of any disease or any symptom of a disease in human beings, or for the regulation of the functional states of the human body, composed solely of (i) any Chinese herbal medicines; (ii) any materials of herbal, animal or mineral origin customarily used by the Chinese, which should be documented in Chinese medicine classics or bibliographies, including but not limited to Pharmacopeia; or (iii) any medicine and materials referred to above, which is formulated in a finished dose form and is generally known or claimed to be used for the diagnosis, treatment, prevention or alleviation of any disease or for the regulation of the functional states of human body.
Section 119 of the HKCMO provides that no person shall sell, import or possess any pCm unless the pCm is registered with the Chinese Medicines Board. Application for registration of a pCm shall be submitted to the Department of Health Chinese Medicines Board in the manner prescribed in section 121 of the HKCMO. Pursuant to section 120 of the HKCMO, the application for registration of any pCm shall be made by the manufacturer of the pCm manufactured in Hong Kong, or by the importer or local representative or agent of the manufacturer of pCm manufactured outside Hong Kong.
Any person who contravenes section 119 of the HKCMO commits an offense and is liable to a maximum fine of HK$100,000 and imprisonment for two years.
50
Manufacture, Sale and Distribution of TCM Products and Chinese Healthcare Products
The HKCMO provides that manufacturers and traders in pCm shall obtain a license issued by the Chinese Medicines Board. Section 131 of the HKCMO provides that no person shall manufacture any pCm, whether registered or not, without a manufacturer license, or at any place other than the premises specified in such license.
Any person who contravenes section 131 or 134 of the HKCMO commits an offense and is liable to a maximum fine of HK$100,000 and imprisonment for two years.
Labeling Requirements and Package Inserts for Chinese Healthcare Products
The Chinese Medicines Regulation (as defined below) is not a PRC legislation but is a Hong Kong legislation. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Chinese Medicines Regulation in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Food and Health Bureau, the Department of Health and etc., as well as the Chinese Medicine Council of Hong Kong, have the implementation rights of Chinese Medicines Regulation. The Chinese Medicine Council of Hong Kong is the main regulator of Chinese Medicines Regulation.
Sections 143 and 144 of the HKCMO provide that a pCm shall not be sold or possessed for the purpose of selling in Hong Kong unless the package of the product is labeled in the prescribed manner and contains a package insert which complies with the prescribed requirements. Pursuant to Regulations 26 and 28 of the Chinese Medicines Regulation (Chapter 549F of the Laws of Hong Kong) (the “Chinese Medicines Regulation”), all pCm shall be properly labeled and attached with package inserts. The label on a package of pCm shall include the following particulars: the name of the medicine; the name of each active ingredient used (if the pCm is composed of three or more kinds of active ingredients, the names of more than half of the active ingredients are required); the registration number on the certificate of registration; the holder of the registration certificate or the manufacturer (if the package is the outermost one, the name of the holder of the certificate of registration is necessary); the name of the country or territory in which the medicine is produced; the packing specification; dosage and method of usage; expiry date; and batch number. The package insert of the pCm shall include the following particulars: the name of the medicine; the name and quantity of each active ingredient used (if the pCm is composed of three or more kinds of active ingredients, the names and quantities of more than half of the active ingredients are required); the name of the holder of certificate of registration or the manufacturer; the dosage and method of usage; functions or pharmacological action; storage instructions; and packing specification. As for the indications, contra-indications, side effects, toxic effects and precautions, they should be included on the package insert as far as practicable.
Any person who contravenes section 143 or 144 of the HKCMO commits an offense and is liable to a maximum fine of HK$100,000 and imprisonment for two years.
Food Safety Ordinance
Food Safety Ordinance (Chapter 612 of the Laws of Hong Kong) (the “Food Safety Ordinance”) establishes a registration scheme for food importers and food distributors, to require the keeping of records by persons who acquire, capture, import or supply food and to enable food import controls to be imposed. Food Safety Ordinance is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Food Safety Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Food and Health Bureau, the Food and Environmental Hygiene Department and etc., has the implementation rights of the Food Safety Ordinance. The Food and Environmental Hygiene Department is the main regulator of the Food Safety Ordinance. As some of our Chinese healthcare products which are non-pCm fall within the definition of food, our company is subject to the regulations under the Food Safety Ordinance.
Food Safety Ordinance may be applicable since we cannot determine whether the ultimate product(s) to be commercialized would be classified by the regulatory body as Chinese herbal medicines, healthcare products, food for ordinary consumption, or otherwise. The precise content of the standardized TCM formulae candidates are subject to further changes and development along with our second efficacy trial. Accordingly, the precise ingredients of TCM products or product candidates will be determined at a later stage of our research and development.
51
Registration as Food Importer or Distributor
Sections 4 and 5 of the Food Safety Ordinance require any person who carries on a food importation business or food distribution business to register with the Food and Environmental Hygiene Department of Hong Kong as a food importer or food distributor. Any person who does not register but carries on a food importation or distribution business, without reasonable excuse, commits an offense and is liable to a maximum fine of HK$50,000 and imprisonment for six months.
Record-keeping Requirement relating to Supply of Food
Section 24 of the Food Safety Ordinance provides that a person who, in the course of business, supplies food in Hong Kong by wholesale must record the following information about the supply: (i) the date the food was supplied; (ii) the name and contact details of the person to whom the food was supplied; (iii) the total quantity of the food; and (iv) a description of the food. Such record shall be made under this section within 72 hours after the time the supply took place.
Any person, who fails to comply with the record-keeping requirement without reasonable excuse or knowingly or recklessly includes in the record information that is false in a material particular, commits an offense and is liable to a maximum fine of HK$10,000 and imprisonment for three months.
Import and Export Ordinance
The Import and Export Ordinance (Chapter 60 of the Laws of Hong Kong) (the “Import and Export Ordinance”) is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Import and Export Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Customs and Excise Department, the Trade and Industry Department and etc., has the implementation rights of the Import and Export Ordinance. The Customs and Excise Department and the Trade and Industry Department are the main regulators of the Import and Export Ordinance.
Pursuant to sections 6C and 6D of the Import and Export Ordinance and Schedules 1 and 2 to the Import and Export (General) Regulations (Chapter 60A of the Laws of Hong Kong), any person who imports or exports any of the Chinese herbal medicines set out in Schedule 1 to the HKCMO and five specific types in Schedule 2 to the HKCMO (namely Flos Campsis (淩霄花), Processed Radix Aconiti (製川烏), Processed Radix Aconiti Kusnezoffii (製草烏), Radix Clematidis (威靈仙) and Radix Gentianae (龍膽)) as well as any pCm under the HKCMO shall apply for an import or export license.
Any person importing or exporting of the aforesaid Chinese herbal medicines and pCm without an import or export license commits an offense and is liable to a fine of HK$500,000 and imprisonment for two years, or on conviction on indictment to a fine of HK$2,000,000 and imprisonment for seven years.
Public Health and Municipal Services Ordinance
The Public Health and Municipal Services Ordinance (Chapter 132 of the Laws of Hong Kong) (the “Public Health Ordinance”) is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Public Health Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Food and Health Bureau, the Home Affairs Bureau, the Drainage Services Department, the Food and Environmental Hygiene Department, the Leisure and Cultural Services Department, the Department of Health, the Lands Department, the Buildings Department, etc., has the implementation rights of the Public Health Ordinance. The Food and Environmental Hygiene Department is the main regulator of the Public Health Ordinance.
52
The legal framework for food safety control in Hong Kong is set out in Part V of the Public Health Ordinance and the relevant sub-legislations thereunder. The Public Health Ordinance requires the manufacturers and sellers of food to ensure that their products are fit for human consumption and comply with the requirements in respect of food safety, food standards and labeling. As some of our Chinese healthcare products which are non- pCm fall within the definition of food, our company is subject to the regulations under the Public Health Ordinance.
Section 50 of the Public Health Ordinance prohibits the manufacture, advertising and sale in Hong Kong of food or drugs that are injurious to health. Any person who fails to comply with this section commits an offence and is liable to a maximum fine of HK$10,000 and imprisonment for three months.
Pursuant to section 52 of the Public Health Ordinance, subject to the statutory defenses set out under section 53 of the Public Health Ordinance, where a seller sells to the prejudice of a purchaser any food or drug which is not of the nature, substance or quality of the food or drug demanded by the purchaser, the seller commits an offense and is liable to a maximum fine of HK$10,000 and imprisonment for three months.
Pursuant to section 54 of the Public Health Ordinance, any person who sells or offers for sale any food intended for, but unfit for, human consumption, or any drug intended for use by human but unfit for the purpose, commits an offense and is liable to a maximum fine of HK$50,000 and imprisonment for six months.
Section 61(1) of the Public Health Ordinance provides that it shall be an offense for any person who gives with any food or drug sold by him/her or displays with any food or drug exhibited for sale by him/her any label which falsely describes the food or drug or is calculated to mislead as to its nature, substance or quality. Furthermore, pursuant to section 61(2) of the Public Health Ordinance, it shall be an offense if any person publishes or is a party to the publication of an advertisement falsely describing any food or drug or is likely to mislead as to the nature, substance or quality of any food or drug. Any person who commits an offense under this section is liable to a maximum fine of HK$50,000 and imprisonment for six months.
Food and Drugs (Composition and Labelling) Regulations
Food and Drugs (Composition and Labelling) Regulations (Chapter 132W of the Laws of Hong Kong) (the “Food and Drugs Regulations”), a subsidiary legislation under the Public Health Ordinance, regulates the advertising and labeling of food. Food and Drugs Regulations is not a PRC legislation but is a Hong Kong legislation. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Food and Drugs Regulations in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Food and Environmental Hygiene Department, etc., has the implementation rights of the Food and Drugs Regulations. The Food and Environmental Hygiene Department is the main regulator of the Food and Drugs Regulations.
Regulation 3 of the Food and Drugs Regulations provides that the manufacturing of foods and drugs shall be up to the standards as specified under Schedule 1 to the Food and Drugs Regulations. Any person who advertises for sale, sells or manufactures for sale any food or drug which does not conform to the relevant requirements as to composition prescribed in Schedule 1 to the Food and Drugs Regulations commits an offense and is liable to a fine of HK$50,000 and imprisonment for six months.
Regulation 4A of the Food and Drugs Regulations demands all pre-packaged food and products sold by the Group (except for those listed in Schedule 4 to the Food and Drugs Regulations) to be marked and labeled in the manner prescribed in Schedule 3 to the Food and Drugs Regulations. Schedule 3 to the Food and Drugs Regulations contains labeling requirements in respect of stating the product’s name or designation, ingredients, “best before” or “use by” date, special conditions for storage or instruction for use, manufacturer’s or packer’s name and address, and quantity, weight or volume, and also includes requirements on the appropriate language or languages for marking or labeling of prepackaged food. Any person who contravenes such requirements commits an offense and is liable to a fine of HK$50,000 and imprisonment for six months.
53
Pursuant to regulation 4B of the Food and Drugs Regulations, prepackaged food sold by the Group should be marked or labeled with its energy value and nutrient content in the manner prescribed in Part 1 of Schedule 5 to the Food and Drugs Regulations, and nutrition claims, if any, made on the label of the product or in any advertisement for the product should comply with Part 2 of Schedule 5 to the Food and Drugs Regulations. Contravention of those requirements may result in a conviction liable to a maximum fine of HK$50,000 and imprisonment for six months.
Trade Marks Ordinance
Trade Marks Ordinance (Chapter 559 of the Laws of Hong Kong) (the “Trade Marks Ordinance”) provides for the registration of trademarks, the use of registered trademarks and related matters. As Hong Kong provides territorial protection for trademarks, trademarks registered in other countries or regions are not automatically entitled to protection in Hong Kong. In order to enjoy protection by the laws of Hong Kong, trademarks shall be registered with the Trade Marks Registry of the Intellectual Property Department under the Trade Marks Ordinance and the Trade Marks Rules (Chapter 599A of the Laws of Hong Kong) (the “Trade Marks Rules”).
Trade Marks Ordinance is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Trade Marks Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Intellectual Property Department and etc., has the implementation rights of the Trade Marks Ordinance. The Intellectual Property Department is the main regulator of the Trade Marks Ordinance.
Section 10 of the Trade Marks Ordinance provides that a registered trademark is a property right acquired through due registration under the Trade Marks Ordinance, through which the owner of a registered trademark is entitled to the statutory rights provided thereunder.
By virtue of section 14 of the Trade Marks Ordinance, the owner of a registered trademark is conferred exclusive rights in the trademark. The rights of the owner in respect of the registered trademark come into existence from the date of the registration of the trademark. Pursuant to section 48 of the Trade Marks Ordinance, the registration date is the filing date of the application for registration.
Subject to the exceptions under sections 19 to 21 of the Trade Marks Ordinance, any use of the trademark by third parties without the consent of the owner is an infringement of the trademark. Section 18 of the Trade Marks Ordinance further specifies the conducts which amount to infringement of the registered trademark. In event that infringement by any third party occurs, the owner of the registered trademark is entitled to remedies under the Trade Marks Ordinance, such as infringement proceedings under sections 23 and 25 of the Trade Marks Ordinance.
Trademarks which are not registered under the Trade Marks Ordinance and the Trade Marks Rules may still be protected by the common law action of passing off, which requires proof of the owner’s reputation in the unregistered trademark and that use of the trademark by third parties will cause damage to the owner.
Sale of Goods Ordinance
Sale of Goods Ordinance (Chapter 26 of the Laws of Hong Kong) (the “Sale of Goods Ordinance”) provides, inter alia, that where a seller sells goods in the course of a business, there is an implied condition that (i) where the goods are purchased by description, the goods shall correspond with the description; (ii) the goods supplied are of merchantable quality; and (iii) the goods shall be fit for the purpose for which they are purchased. Otherwise, a buyer has the right to reject the defective goods unless he or she has a reasonable opportunity to examine the goods. A breach of the implied term may give rise to a civil action for breach of contract by the customers. However, no criminal liability arises from such breach of implied term.
Sale of Goods Ordinance is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Sale of Goods Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. As the Sale of Goods Ordinance only provides for civil courses of action, there is no regulator or implementer for this ordinance.
54
Employment Ordinance (Chapter 57 of the Laws of Hong Kong)
The Employment Ordinance (Chapter 57 of the Laws of Hong Kong) (the “Employment Ordinance”) is an ordinance enacted for, amongst other things, the protection of the wages of employees and the regulation of the general conditions of employment and employment agencies. Employment Ordinance is not a PRC legislation but is Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the Employment Ordinance in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Labor Department, the Department of Health, the Labor Tribunal, etc., has the implementation rights of the Employment Ordinance. The Labor Department is the main regulator of the Employment Ordinance.
Under the Employment Ordinance, an employee is generally entitled to, amongst other things, notice of termination of his or her employment contract; payment in lieu of notice; maternity protection in the case of a pregnant employee; not less than one rest day in every period of seven days; severance payments or long service payments; sickness allowance; statutory holidays or alternative holidays; and paid annual leave of up to 15 days depending on the period of employment.
Employees’ Compensation Ordinance (Chapter 282 of the Laws of Hong Kong)
The Employees’ Compensation Ordinance (Chapter 282 of the Laws of Hong Kong) (the “ECO”), is an ordinance enacted for the purpose of providing for the payment of compensation to employees injured in the course of employment. The ECO is not a PRC legislation but is a Hong Kong ordinance. As with all other Hong Kong legislations, according to the laws of Hong Kong, the Legislative Council of Hong Kong has the power and function to amend the ECO in accordance with the provisions of the Hong Kong Basic Law and legal procedures. The Hong Kong Government, including but not limited to the Department of Justice, the Hong Kong Police Force, the Labor Department, the Department of Health and etc., has the implementation rights of the ECO. The Labor Department is the main regulator of the ECO.
As stipulated by the ECO, no employer shall employ any employee in any employment unless there is in force in relation to such employee a policy of insurance issued by an insurer for an amount not less than the applicable amount specified in the Fourth Schedule of the ECO in respect of the liability of the employer. According to the Fourth Schedule of the ECO, the insured amount shall be not less than HK$100,000,000 per event if a company has no more than 200 employees. Any employer who contravenes this requirement commits a criminal offense and is liable on conviction to a fine and imprisonment. An employer who has taken out an insurance policy under the ECO is required to display a prescribed notice of insurance in a conspicuous place on each of its premises where any employee is employed.
C. Organizational Structure
See “Item 4. Information on the Company – A. History and Development of the Company.”
D. Property, Plants and Equipment
See “Item 4. Information on the Company – B. Business Overview – Our Leases.”
Item 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
55
Item 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 20-F. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Item 3. Key Information—D. Risk Factors” or in other parts of this Annual Report on Form 20-F.
| A. | Operating Results |
Overview
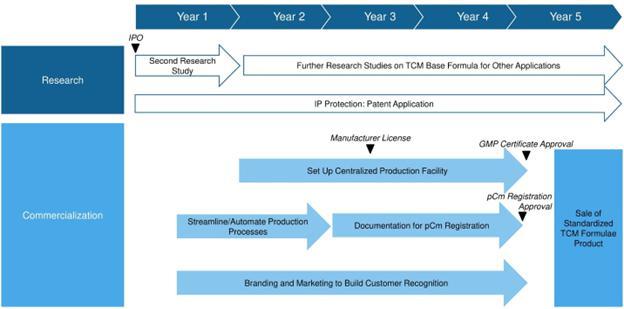
We are a holding company incorporated on October 30, 2014, under the laws of the Cayman Islands, and conduct our business in Hong Kong through our wholly-owned subsidiaries, Regencell Bioscience Limited, a company incorporated in Hong Kong on May 12, 2015, Regencell Limited, a company incorporated in Hong Kong on November 20, 2014, and Regencell Bioscience North America Limited, a company incorporated in the British Virgin Islands on April 25, 2022, and a 60% joint venture, Regencell Bioscience Asia Limited, a company incorporated in Hong Kong on September 17, 2021. We ceased the business of Regencell Bioscience Asia Limited on December 31, 2023 and the deregistration was in progress. The disposition of the joint venture did not have a material impact on our ongoing results of operations of financial results. We are an early-stage bioscience company that focuses on research, development, and commercialization of TCM for the treatment of neurocognitive disorders and degeneration, specifically ADHD and ASD. Our goal is to save and improve the lives of ADHD and ASD patients, their families and caregivers and become a market leader for the best natural and holistic treatment globally.
Our TCM formulae candidates are derived from a TCM base formula and an adjustable formula developed by our TCM Practitioner based on his TCM brain theory, known as “Sik-Kee Au TCM Brain Theory®”, and has demonstrated reduced severity in patients’ ADHD and ASD conditions, as reflected in lower SKATBT-A3, ATEC, GARS, VADRS and SNAP-IV-26 assessment scores, using the personalized TCM formula in our first research study. The activity and specificity of the TCM base formula have been optimized by the TCM Practitioner in his prior ADHD and ASD treatments. The TCM Practitioner has standardized the adjustable formula into three Fixed Adjusted Formula for mild, moderate and severe ADHD and ASD conditions. Reduced severity in patients’ ADHD and ASD conditions has also been demonstrated as reflected in lower SKATBT-A3, ATEC and VADRS assessment scores, using the standardized TCM formula in our second research study. The TCM brain theory is not recognized in general literature of TCM or elsewhere. However, the TCM Practitioner has prescribed the TCM formula based on his TCM brain theory for over 30 years to treat ADHD, ASD and many neurological illnesses, disorders and degeneration and obtained satisfactory clinical treatment results. Such clinical treatment results are not supported by controlled clinical data or trials.
We aim to launch three standardized liquid-based TCM formulae candidates for mild, moderate and severe ADHD and ASD patients in Hong Kong first and subsequently to other markets as we deem appropriate. We have been headquartered in Hong Kong since inception.
56
The Initial Public Offering
On July 20, 2021, we completed our IPO of 2,300,000 Ordinary Shares, $0.00001 par value per share at an offering price of $9.50 per share, generating gross proceeds of approximately $21.85 million. On August 17, 2021, the underwriter of the IPO exercised its option to purchase 325,000 additional Over-allotment Shares at a price of $9.50 per share. The closing of the sale of the Over-allotment Shares took place on August 19, 2021. The net proceeds of the IPO, including proceeds from the sale of Over-allotment Shares, totaled approximately $22.67 million, after deducting underwriting discounts and other related expenses, of approximately of $2.26 million.
Public Offering Warrants
In connection with and upon closing of the IPO and over-allotment on July 20, 2021 and August 19, 2021, respectively, we issued warrants equal to 2.5% of the shares issued in the IPO, totaling 57,500 units and 8,125 units to the placement agents for the offering. The warrants carry a term of five years, and shall not be exercisable for a period of 180 days from the closing of the IPO and shall be exercisable at a price equal to $10.45 per share. All warrants were issued and exercised.
Financial Operations Overview
Revenue
We have not generated any revenue from the sale of any products, and we do not expect to generate any revenue until we commercialize our standardized TCM formulae products for ADHD and ASD patients in Hong Kong.
Selling and Marketing Expenses
Expenses in marketing were mainly for marketing initiatives and sponsorship with Non-Governmental Organizations (“NGOs”) and institutions that serves families with ADHD and ASD children, who voluntary sign up as qualified patients to be enrolled in our efficacy trial in the future. As we commercialize our TCM formulae products, we expect that we would be hiring a sales and marketing team, engaging external sales and marketing professional services, and media coverage.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, compensation, legal and accounting fees, as well as office rental, computer equipment and software and utilities.
We anticipate that our general and administrative expenses will support our continued research and development activities and costs of operating as a public company. These increases will likely include increased costs related to the hiring of additional personnel and fees to outside consultants, lawyers and accountants, among other expenses. Additionally, we anticipate increased costs associated with being a public company including expenses related to services associated with maintaining compliance with Nasdaq Stock Market listing rules and SEC requirements and investor relations costs.
Research and Development Expenses
Since our inception, our operations have primarily been limited to the research studies of the proprietary TCM formulae. Our research and development expenses to date consist mainly of employee salaries and related benefits, office rental and depreciation.
Research and development activities will continue to be central to our business model. Future research and development expenses will include employee-related expenses, such as salaries, share-based compensation, benefits and travel expense for the research and development personnel that we plan to hire and treatment costs in connection with conducting research studies.
57
Results of Operations
Results of Operations for the Years Ended June 30, 2024 and 2023
The following table sets forth our results of operations for the year ended June 30, 2024 compared to the year ended June 30, 2023:
| For the Year Ended | For the Year Ended | |||||||||||||||
| June 30, | June 30, | Change | Change | |||||||||||||
| 2024 | 2023 | Amount | % | |||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Selling and marketing | $ | 125,427 | $ | 262,664 | $ | (137,237 | ) | (52 | )% | |||||||
| General and administrative | 3,545,066 | 4,429,379 | (884,313 | ) | (20 | )% | ||||||||||
| Research and development | 1,066,233 | 1,581,628 | (515,395 | ) | (33 | )% | ||||||||||
| Total operating expenses | 4,736,726 | 6,273,671 | (1,536,945 | ) | (24 | )% | ||||||||||
| LOSS FROM OPERATIONS | (4,736,726 | ) | (6,273,671 | ) | 1,536,945 | (24 | )% | |||||||||
| OTHER INCOME, NET | 373,505 | 211,342 | 162,163 | 77 | % | |||||||||||
| LOSS BEFORE INCOME TAXES | (4,363,221 | ) | (6,062,329 | ) | 1,699,108 | (28 | )% | |||||||||
| PROVISION FOR INCOME TAXES | - | - | - | - | ||||||||||||
| NET LOSS | (4,363,221 | ) | (6,062,329 | ) | 1,699,108 | (28 | )% | |||||||||
| OTHER COMPREHENSIVE LOSS | ||||||||||||||||
| Foreign currency translation adjustment | 40,892 | (86,658 | ) | 127,550 | (147 | )% | ||||||||||
| COMPREHENSIVE LOSS | (4,322,329 | ) | (6,148,987 | ) | 1,826,658 | (30 | )% | |||||||||
Revenue
We have not generated any revenue from the sale of any products for the years ended June 30, 2024 and 2023, respectively.
Selling and Marketing Expenses
Selling and marketing expenses were $0.1 million and $0.3 million for the years ended June 30, 2024 and 2023, respectively. The expenses were primarily attributable to marketing initiatives and sponsorship with NGOs and institutions that serve families with ADHD and ASD children, who voluntary sign up as qualified patients to be enrolled in our efficacy trial in the future during those periods.
The decreased expenses in selling and marketing expenses in the year ended June 30, 2024 compared with the year ended June 30, 2023 were mainly due to a decrease in the expenses through marketing initiatives.
58
General and Administrative Expenses
General and administrative expenses were $3.5 million and $4.4 million for the years ended June 30, 2024 and 2023, respectively, and were primarily attributable to employee salaries and related benefits, office rental, depreciation, legal, professional fees and consulting services associated with the formation of our company, corporate matters and certain direct and indirect costs associated with services obtained by our company.
The decreased general and administrative expenses in the year ended June 30, 2024 compared with the year ended June 30, 2023 were mainly attributable to (i) approximately $0.4 million of decrease in amortization of share-based compensation for our general and administrative personnel, (ii) approximately $0.2 million of decrease in professional fees, such as legal services and public relations services as we became a public company listed on the Nasdaq Capital Market, (iii) approximately $0.2 million of decrease in payroll mainly due to a decrease in bonus and the termination of a special advisor, and (iv) approximately $0.1 million of decrease in traveling, meetings and others mainly due to less traveling was incurred.
Research and Development Expenses
Research and development expenses were $1.1 million and $1.6 million for the years ended June 30, 2024 and 2023, respectively, and were primarily attributable to employee salaries and related benefits, office rental, depreciation, and other third-party services associated with efficacy trials.
The decreased research and development expenses in the year ended June 30, 2024 compared with the year ended June 30, 2023 were mainly attributable to (i) approximately $0.06 million of decrease in amortization of share-based compensation for our research and development personnel; (ii) approximately $0.1 million of decrease in salaries due to the decrease in the bonus, (iii) approximately $0.2 million of decrease in expenses from TCM formulae and materials for product development, (iv) approximately $0.06 million of decrease in rental expenses and building management fee for rental of office and staff quarters and (iv) approximately $0.08 million of decrease in services fee for research support, testing equipment and others mainly due to more research and development activities incurred during the year.
Other Income, Net
Other income, net was $0.4 million and $0.2 million for the years ended June 30, 2024 and 2023. The other income was primarily attributable to the receipt of government grants in respect of COVID-19-related subsidies (for the year ended June 30, 2023 only), the Employment Support Scheme provided by the Hong Kong Government under the Anti-Epidemic Fund and the interest income generated from short-term investment. The increase in other income was mainly because approximately $0.2 million increase in interest income received from short-term investment and bank deposits during the year ended June 30, 2024 when compared to the year ended June 30, 2023.
Provision for Income Taxes
We did not have any provision for income taxes for the years ended June 30, 2024 and 2023.
Net Loss
As a result of the foregoing, we had net loss of $4.4 million and $6.1 million for the years ended June 30, 2024 and 2023, respectively.
59
Results of Operations for the Years Ended June 30, 2023 and 2022
The following table sets forth our results of operations for the year ended June 30, 2023 compared to that of the year ended June 30, 2022:
| For the Year Ended | For the Year Ended | |||||||||||||||
| June 30, | June 30, | Change | Change | |||||||||||||
| 2023 | 2022 | Amount | % | |||||||||||||
| OPERATING EXPENSES: | ||||||||||||||||
| Selling and marketing | $ | 262,664 | $ | 25,275 | $ | 237,389 | 939 | % | ||||||||
| General and administrative | 4,429,379 | 5,080,341 | (650,962 | ) | (13 | )% | ||||||||||
| Research and development | 1,581,628 | 2,512,154 | (930,526 | ) | (37 | )% | ||||||||||
| Total operating expenses | 6,273,671 | 7,617,770 | (1,344,099 | ) | (18 | )% | ||||||||||
| LOSS FROM OPERATIONS | (6,273,671 | ) | (7,617,770 | ) | 1,344,099 | (18 | )% | |||||||||
| OTHER INCOME, NET | 211,342 | 23,215 | 188,127 | 810 | % | |||||||||||
| LOSS BEFORE INCOME TAXES | (6,062,329 | ) | (7,594,555 | ) | 1,532,226 | (20 | )% | |||||||||
| PROVISION FOR INCOME TAXES | - | - | - | - | ||||||||||||
| NET LOSS | (6,062,329 | ) | (7,594,555 | ) | 1,532,226 | (20 | )% | |||||||||
| OTHER COMPREHENSIVE LOSS | ||||||||||||||||
| Foreign currency translation adjustment | (86,658 | ) | - | (86,658 | ) | N/A | ||||||||||
| COMPREHENSIVE LOSS | (6,148,987 | ) | (7,594,555 | ) | 1,445,568 | (19 | )% | |||||||||
Revenue
We have not generated any revenue from the sale of any products for the years ended June 30, 2023 and 2022, respectively.
Selling and Marketing Expenses
Selling and marketing expenses were $0.3 million and $0.03 million for the years ended June 30, 2023 and 2022, respectively. The expenses were primarily attributable to marketing initiatives and sponsorship with NGOs and institutions that serve families with ADHD and ASD children, who voluntary sign up as qualified patients to be enrolled in our efficacy trial in the future during those periods.
The increased expenses in selling and marketing expenses in the year ended June 30, 2023 compared with the year ended June 30, 2022 were mainly due to an increase in the expenses through marketing initiatives.
60
General and Administrative Expenses
General and administrative expenses were $4.4 million and $5.1 million for the years ended June 30, 2023 and 2022, respectively, and were primarily attributable to employee salaries and related benefits, office rental, depreciation, legal, professional fees and consulting services associated with the formation of our company, corporate matters and certain direct and indirect costs associated with services obtained by our company.
The decreased general and administrative expenses in the year ended June 30, 2023 compared with the year ended June 30, 2022 were mainly attributable to (i) approximately $0.6 million of decrease in amortization of share-based compensation for our general and administrative personnel, (ii) approximately $0.4 million of decrease in professional fees, such as legal services and public relations services as we became a public company listed on the Nasdaq Capital Market, and offset by (iii) approximately $0.3 million of increase in traveling, meetings and others mainly due to more business activities relating to our business expansion.
Research and Development Expenses
Research and development expenses were $1.6 million and $2.5 million for the years ended June 30, 2023 and 2022, respectively, and were primarily attributable to employee salaries and related benefits, office rental, depreciation, and other third-party services associated with efficacy trials.
The decreased research and development expenses in the year ended June 30, 2023 compared with the year ended June 30, 2022 were mainly attributable to (i) approximately $1.2 million of decrease in amortization of share-based compensation for our research and development personnel and offset by (ii) approximately $0.1 million increase in salaries due to the increase in the salaries payments, (iii) approximately $0.05 million of increase in expenses from TCM formulae and materials for product development, (iv) approximately $0.05 million of increase in rental expenses and building management fee for rental of office and staff quarters and (iv) approximately $0.1 million of increase in services fee for research support, testing equipment and others mainly due to more research and development activities incurred during the year.
Other Income, Net
Other income, net was $0.2 million and $0.02 million for the years ended June 30, 2023 and 2022. The other income was primarily attributable to the receipt of government grants in respect of COVID-19-related subsidies, the Employment Support Scheme provided by the Hong Kong Government under the Anti-Epidemic Fund and the interest income generated from short-term investment. The increase in other income was mainly because (i) approximately $0.01 million increase in subsidies received and (ii) approximately $0.17 million increase in interest income received from short-term investment and bank deposits during the year ended June 30, 2023 when compared to the year ended June 30, 2022.
Provision for Income Taxes
We did not have any provision for income taxes for the years ended June 30, 2023 and 2022.
Net Loss
As a result of the foregoing, we had net loss of $6.1 million and $7.6 million for the years ended June 30, 2023 and 2022, respectively.
Taxation
Cayman Islands and British Virgin Islands
We are incorporated in the Cayman Islands and our primary business operations are conducted through our subsidiaries. Under the current laws of the Cayman Islands and the British Virgin Islands, we are not subject to tax on income or capital gains.
61
Hong Kong Profits Taxation
Our subsidiaries incorporated in Hong Kong were subject to 16.5% Hong Kong profits tax on their taxable income assessable profits generated from operations arising in or derived from Hong Kong for the year of assessment of 2022/2023 and 2023/2024. As from year of assessment of 2020/2021 onwards, Hong Kong profits tax rates are 8.25% on assessable profits up to HK$2,000,000, and 16.5% on any part of assessable profits over HK$2,000,000.
In December 2022, a refined Foreign-sourced Income Exemption (“FSIE”) regime was published in Hong Kong and took effect from January 1, 2023. Under the new FSIE regime, certain foreign sourced income would be deemed as being sourced from Hong Kong and chargeable to Hong Kong Profits Tax, if the recipient entity fails to meet the prescribed exception requirements. Certain dividends, interests, disposal gains and intellectual property income, if any, received by us and our Hong Kong subsidiaries will be subject to the new tax regime. Based on our preliminary analysis, we do not believe this legislation will have a material impact on our financial statements. We will monitor the regulatory developments and continue to evaluate the impact, if any. In addition, payments of dividends from our Hong Kong subsidiary to us are not subject to any withholding tax in Hong Kong.
| B. | Liquidity and Capital Resources |
In accordance with Accounting Standards Update (ASU) 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (Subtopic 205-40), we have evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that the consolidated financial statements are issued.
We have incurred recurring negative cash flows since inception and have funded our operations primarily from shareholder loans and proceeds from IPO, including over-allotments in the IPO. Prior to the completion of IPO, we received funding in the form of shareholder loans to support our operating needs, which was provided by our CEO.
With the completion of its IPO and debt conversion, our ability to continue as a going concern was greatly improved. As of June 30, 2024, we had a total of $8.0 million in cash and short-term investment. Our cash and short-term investment consist primarily of cash on hand and time deposits placed with banks, which are unrestricted as to withdrawal and use.
We believe that our current cash and short-term investments balance as of June 30, 2024 is sufficient to fund our operating activities, capital expenditures and other obligations for at least the next twelve months from the date the audited financial statements were issued. However, we may decide to enhance our liquidity position or increase our cash reserve for future expansions and acquisitions through additional capital and/or finance funding. The issuance and sale of additional equity would result in further dilution to our shareholders.
The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Accordingly, the consolidated financial statements have been prepared on a basis that assumes we will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
The following summarizes the key components of our cash flows for the years ended June 30, 2024, 2023 and 2022.
| For the Years Ended June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Net cash used in operating activities | $ | (4,001,687 | ) | $ | (4,962,949 | ) | $ | (5,267,055 | ) | |||
| Net cash provided by (used in) investing activities | 5,242,624 | 58,978 | (10,780,876 | ) | ||||||||
| Net cash provided by financing activities | 102,282 | 134,913 | 22,405,516 | |||||||||
| Effect of exchange rate on cash | 53,221 | (83,145 | ) | - | ||||||||
| Net change in cash | $ | 1,396,440 | $ | (4,852,203 | ) | $ | 6,357,585 | |||||
| Cash, beginning of year | 1,564,795 | 6,416,998 | 59,413 | |||||||||
| Cash, end of year | $ | 2,961,235 | $ | 1,564,795 | $ | 6,416,998 | ||||||
62
Cash Flows
Operating Activities
Fiscal Years Ended June 30, 2024 and 2023
For the years ended June 30, 2024 and 2023, net cash used in operating activities were $4.00 million and $4.96 million, respectively. Net cash used in operating activities was primarily attributable to rental of offices and staff quarters, the hiring of our executive staff, research and development, ramped up marketing efforts and general and administrative activities.
Fiscal Years Ended June 30, 2023 and 2022
For the years ended June 30, 2023 and 2022, net cash used in operating activities were $4.96 million and $5.27 million, respectively. Net cash used in operating activities was primarily attributable to rental of offices and staff quarters, the hiring of our executive staff, research and development, ramped up marketing efforts and general and administrative activities.
Investing Activities
Fiscal Years Ended June 30, 2024 and 2023
For the years ended June 30, 2024 and 2023, net cash provided by investing activities were $5.24 million and $0.06 million, respectively. Net cash provided by investing activities during the year ended June 30, 2024 was primarily attributable to the maturity of short-term investment, partially offset by reinvestment.
Fiscal Years Ended June 30, 2023 and 2022
For the years ended June 30, 2023 and 2022, net cash provided by (used in) investing activities were $0.06 million and $10.8 million, respectively. Net cash provided by investing activities during the year ended June 30, 2023 was primarily attributable to the mature of short-term investment. On the other hand, the net cash used in investing activities during the year ended June 30, 2022 was primarily attributable to placement of $10 million in a short-term investment; renovation of our new office space and purchase of property and equipment.
Financing Activities
Fiscal Years Ended June 30, 2024 and 2023
For the years ended June 30, 2024 and 2023, net cash from financing activities were $0.1 million and $0.1 million, respectively. Net cash from financing activities was primarily attributable to capital contribution from non-controlling interest of subsidiary.
Fiscal Years Ended June 30, 2023 and 2022
For the years ended June 30, 2023 and 2022, net cash from financing activities were $0.1 million and $22.4 million, respectively. Net cash from financing activities was primarily attributable to capital contribution from non-controlling interest of subsidiary and, for the fiscal year ended June 30, 2022, net proceeds from initial public offerings.
63
Contractual Obligations
The following table summarizes our minimum lease payments under operating lease in future periods as of June 30, 2024:
| Year Ended June 30, 2024 | ||||
| For the year ending June 30, | ||||
| 2025 | $ | 62,792 | ||
| 2026 | 25,128 | |||
| 2027 | - | |||
| Total minimum lease payments | $ | 87,920 | ||
| Less: Amount representing interest | (2,179 | ) | ||
| Present value of net minimum lease payments | $ | 85,741 | ||
Capital Expenditures
For the years ended June 30, 2024, 2023 and 2022, we had capital expenditures of $5.4 thousand, $0.03 million and $0.78 million, respectively, in relation to our property and equipment. Subsequent to June 30, 2024 and as of the date of this Annual Report, we did not purchase any material equipment for operational use. We do not have any other material commitments to capital expenditures as of June 30, 2024.
Holding Company Structure
Regencell Bioscience Holdings Limited is a holding company incorporated on October 30, 2014 under the laws of the Cayman Islands and has no substantive operations other than holding all of the issued and outstanding shares of our subsidiaries, namely Regencell Bioscience Limited incorporated in Hong Kong, Regencell Limited incorporated in Hong Kong and Regencell Bioscience North America Limited incorporated in the British Virgin Islands. We conduct our operations primarily through our subsidiaries. As a result, our ability to pay dividends depends upon, among others, dividends paid by our subsidiaries. If our subsidiaries or any newly formed subsidiaries incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to us.
Off-Balance Sheet Arrangements
As of June 30, 2024, we did not have any off-balance sheet arrangements with unconsolidated entities or persons that had or were reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, cash requirements or capital resources; and we did not enter into any guarantees; retained or contingent interests in assets transferred; contractual arrangements that support the credit, liquidity or market risk for transferred assets; obligations that arise or could arise from variable interests held in an unconsolidated entity; or obligations related to derivative instruments that are both indexed to and classified in our own equity, or not reflected in the statement of financial position.
64
| C. | Research and development, Patents and License, etc. |
Research and development expenses consist of costs incurred by our company for the discovery and development of our product candidates. Research and development costs include, but not limited to, payroll and personnel expenses including stock-based compensation, research studies supplies, fees for efficacy trial services, consulting costs, and allocated overhead, including rent, equipment, and utilities.
| D. | Trend Information |
Other than as disclosed elsewhere in this Annual Report on Form 20-F, for the year ended June 30, 2024, we are not aware of any trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause reported financial information not necessarily to be indicative of future operating results or financial condition.
| E. | Critical Accounting Estimates |
Critical accounting estimates are those estimates made in accordance with generally accepted accounting principles that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on the financial condition or results of operations of the registrant.
Share-based compensation
We measure all share option grants to our directors, executive officers and other employees based on their fair value on the date of the grant and recognize the corresponding compensation expense of those awards using the straight-line method over the requisite service period, which is generally the vesting period of the respective award. Forfeitures are accounted for as they occur.
We classify share-based compensation expense in our statements of operations in the same way the award recipient’s payroll costs are classified or in which the award recipient’s service payments are classified.
We estimate the fair value of each share option grant using the Black Scholes Model and/or the Binomial Model, which involves key assumptions of expected volatility, risk-free interest rate, exercise multiples, expected dividend yield, life of options, and fair value of underlying ordinary shares.
For the years ended June 30, 2024, 2023 and 2022, the Company had share-based compensation expenses of approximately $0.4 million, $0.9 million, and $2.7 million, respectively. See Note 7 to our audited financial statements included elsewhere in this Annual Report for further information concerning the assumptions we used in in determining share-based compensation.
Deferred tax assets
We follow the liability method of accounting for income taxes in accordance with ASC 740, Income Taxes (“ASC 740”). Under this method, deferred income taxes are recognized when temporary differences exist between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the cumulative earnings and projected future taxable income in making this assessment. Recovery of substantially all of the Company’s deferred tax assets is dependent upon the generation of future income, exclusive of reversing taxable temporary differences. Based upon historical operating results and projections for future taxable income, management provided full valuation allowance for the deferred tax assets for the years ended June 30, 2024 and 2023. As of June 30, 2024 and 2023, tax loss carry-forward amounted to approximately $17 million and $14 million, respectively can be carried forward indefinitely.
65
Recent Accounting Pronouncements
See “Note 2 – Summary of significant accounting policies – Recently issued accounting pronouncements” in the accompanying notes to consolidated financial statements included elsewhere in this Annual Report.
We are an “emerging growth company” as defined under the federal securities laws and, as such, will be subject to reduced public company reporting requirements. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and acknowledge such election is irrevocable pursuant to Section 107 of the JOBS Act. As a result of our election, our financial statements may not be comparable to those of companies that comply with public company effective dates.
Safe Harbor
This Annual Report on Form 20-F contains forward-looking statements. These statements are made under the “safe harbor” provisions of Section 21E of the Exchange Act. These forward-looking statements can be identified by terminology such as “will,” “expects,” “anticipates,” “future,” “intends,” “plans,” “believes,” “estimates,” “may,” “intend,” “is currently reviewing,” “it is possible,” “subject to” and similar statements. Among other things, the sections titled “Item 3. Key Information—D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects” in this Annual Report on Form 20-F, as well as our strategic and operational plans, contain forward-looking statements. We may also make written or oral forward-looking statements in our filings with the SEC, in our Annual Report to shareholders, in press releases and other written materials and in oral statements made by our officers, directors or employees to third parties. Statements that are not historical facts, including statements about our beliefs and expectations, are forward-looking statements and are subject to change, and such change may be material and may have a material and adverse effect on our financial condition and results of operations for one or more prior periods. Forward-looking statements involve inherent risks and uncertainties. A number of important factors could cause actual results to differ materially from those contained, either expressly or impliedly, in any of the forward-looking statements in this Annual Report on Form 20-F. All information provided in this Annual Report on Form 20-F and in the exhibits is as of the date of this Annual Report on Form 20-F, and we do not undertake any obligation to update any such information, except as required under applicable law.
66
Item 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
Directors and Executive Officers
The following table sets forth our executive officers and directors as of the date hereof, as well as their ages and the positions held by them:
| Name | Age | Position | ||
| Yat-Gai Au | 52 | Chairman, Director and Chief Executive Officer | ||
| James Wai Hong Chung | 47 | Director, Chief Operating Officer and Chief Strategy Officer | ||
| Yat-Pui Au | 50 | Chief Business Officer | ||
| Evana Yee Wah Hui (1) | 52 | Independent Director | ||
| Paul J. Niewiadomski (2) | 54 | Independent Director | ||
| Dr. Wing Yan (William) Lo (3) | 63 | Independent Director |
| (1) | Chair of the Compensation Committee. |
| (2) | Chair of the Nominating and Corporate Governance Committee. |
| (3) | Chair of the Audit Committee. |
67
The following is a brief biography of each of our executive officers and directors:
Yat-Gai Au
Mr. Yat-Gai Au is our Chief Executive Officer and has been our director since 2014. He began his career in investment banking at Deutsche Bank and ING Barings and was one of the core team members that won Merger and Acquisitions deal of the year awards in Asia from 1998 to 1999 comprising over $4 billion worth of deals. He is an active property, technology and biotech investor. He funds and participates in several charity events for thousands of underprivileged families and elderlies in Hong Kong and the U.S. During the COVID-19 outbreak, he donated over 200,000 masks to hospitals, elderlies and needy families. In September 2018, Mr. Au set up Regencell Foundation Limited to further his charitable endeavors. Mr. Yat-Gai Au is the son of the TCM Practitioner, Mr. Sik-Kee Au. Mr. Au graduated from University of California, Berkeley with a Bachelor of Science degree from the Haas School of Business.
James Wai Hong Chung
Mr. James Wai Hong Chung has been our director since December 28, 2023 our Chief Operating Officer (“COO”) since August 2021 and Chief Strategy Officer since October 2020. He has joined Regencell Bioscience Limited as senior vice president since July 2015. He brings 15 years of experience in various leading roles. Prior to joining Regencell, Mr. Chung managed the Asia equities and derivatives electronic trading platform at Investment Technology Group, where he gained extensive banking and finance experience. Prior to that, he worked at Sungard Financial Systems, providing front to back trading and risk management software solutions for banks. He also gained experiences in different roles from sales and accounting management, project management, technology and business analyst when he worked at a startup hedge fund IT consulting business. Mr. Chung started his career as a telecommunication engineer at Hutchison Global Crossing and eventually led a small team of engineers. Mr. Chung is a graduate of Quantitative Finance from the Management Science and Engineering Department at Stanford University and Telecommunications Technology from British Columbia Institute of Technology.
Yat-Pui Au
Mr. Yat-Pui Au has been our Chief Business Officer (“CBO”) since February 2023. He has joined Regencell Bioscience Limited as senior vice president since October 2021. He has more than 24 years of experience in strategic management and business operations. Prior to joining Regencell, he worked at various leading roles in the physical security and property management sectors, where he implemented digital and cloud-based CRM solutions, completed major technology projects for governmental/institutional/private sectors, and streamlined business operations across marketing, finance, IT, HR and operations departments. Mr. Au started his career as a consultant analyst for Computer Sciences Corporation for IT/MIS integrations and deployment. Mr. Yat-Pui Au is Mr. Yat-Gai Au’s brother. Mr. Au obtained his Bachelor’s degree in Legal Studies from UC Berkeley.
Evana Yee Wah Hui
Ms. Evana Yee Wah Hui has been our independent director and member of audit committee, compensation committee and nominating and corporate governance committee since July 2021. She has over 20 years of retail and brand management experience. Throughout her 18-year career at the Dairy Farm Company Limited, a leading pan-Asian retailer, she managed various segments of the business both at the regional and local level. She headed the regional category development team of the Health& Baby categories across the Greater China and Asian regions covering nine markets, implementing store layout/display, category development and management strategies. Furthermore, she was responsible for the sales and profitability of a diverse range of categories with experience in product creation and development, brand building, and customer loyalty. She also spearheaded the launch of both the GNC and Boots brands in Hong Kong. Prior to that, she spent three years developing and implementing market research at Nielsen, where her major clients included Unilever, Coca-Cola, HSBC, MSD, and L’Oreal. Ms. Hui participated in charitable endeavors, such as promoting mental health awareness in the community and holding workshops to promote self-awareness and reduce stigma in local high schools, co-organized by the HK Hospital Authority. She holds an MBA from University of Southern California and graduated with a Bachelor of Arts, Mathematics (Honors) from Mills College.
68
Paul J. Niewiadomski
Mr. Paul J. Niewiadomski has been our independent director and member of audit committee, compensation committee and nominating and corporate governance committee since July 2021. He is a partner in Lubin Olson& Niewiadomski LLP’s Business, Finance and Workouts Practice Groups. He represents clients in a broad range of business transactions. His practice encompasses acquisitions, dispositions, joint ventures, and financing. He has advised both publicly traded and private companies on business matters. Mr. Niewiadomski served on the boards of several non-profit, for profit and civic organizations and is a member of the Urban Land Institute (San Francisco) and NAIOP (San Francisco Bay Area). He has spoken at a wide range of seminars and conferences on business issues for Marcus & Millichap, California County Counsels’ Association, California Mortgage Association, and other organizations. Mr. Niewiadomski is recognized by Thomson Reuters as a Northern California “Super Lawyer” and was selected by his peers for the inclusion of the 2018, 2019 and 2020 Editions of The Best Lawyers in America. He is a Juris Doctor graduate from University of Michigan, Master of Laws from New York University and a Bachelor of Business Administration from Western Michigan University (Magna Cum Laude).
Dr. Wing Yan (William) Lo
Dr. Wing Yan (William) Lo has been our independent director and member of audit committee, compensation committee and nominating and corporate governance committee since December 2021. He has over three decades of biopharma, academic medicine, corporate governance, and strategic advisory experience. Dr. Lo graduated from Cambridge University with a Master of Philosophy Degree in Pharmacology and a Ph.D. Degree in Molecular Neuroscience. Dr. Lo started his career in McKinsey & Company Inc. as a management consultant and held senior positions in China Unicom, Hongkong Telecom, Citibank HK, I.T Limited, South China Media Group and Kidsland International Holdings Limited in the past. Dr. Lo currently serves as an independent non-executive director of a number of public companies listed on the Main Board of The Stock Exchange of Hong Kong Limited (“HKSE”); Dr. Lo is an independent non-executive director of Television Broadcasts Ltd (HKSE stock code: 511), OCI International Holdings Limited (HKSE stock code: 329), CSI Properties Limited (HKSE stock code: 497), Jingrui Holdings Limited (HKSE stock code: 1862), and Oshidori International Holdings Limited (HKSE stock code: 622). Dr. Lo was also an independent non-executive director of Nam Tai Property Inc. (NYSE stock code: NTP) which was formerly listed on the New York Stock Exchange (“NYSE”). Dr. Lo was appointed a Justice of Peace (JP) of the Government of the Hong Kong Special Administrative Region and is the founding governor of the Charles K. Kao Foundation for Alzheimer’s Disease and the ISF Academy as well as the present chairman of Junior Achievement HK.
Principal Financial Officer
Michelle Chan is our financial controller who joined us since November 2021. Ms. Chan has assumed the responsibilities of the chief financial officer since April 2022 and is currently our principal financial officer.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
B. Compensation
Executive Compensation
For the year ended June 30, 2024, we paid an aggregate of $0.78 million in cash to our directors and executive officers. We are not required under Cayman Islands law to disclose, and we have not otherwise disclosed, the compensation of our directors and executive officers on an individual basis. We have not set aside or accrued any amounts to provide pension, retirement or other similar benefits to our directors and executive officers. Our subsidiaries in Hong Kong are required by law to make contributions equal to certain percentages of each qualifying employee’s salary to the Mandatory Provident Fund Scheme.
During the years ended June 30, 2024 and 2023, we did not pay any salary and bonus to Mr. Yat-Gai Au, our CEO, for services rendered to us. Pursuant to the employment agreement, as amended and restated, we entered into with Mr. Yat-Gai Au, Mr. Au shall receive an annual base salary of $1, and such compensation is subject to annual review and adjustment by our board of directors. The CEO cannot draw his compensation more than $1 until we reach a market capitalization of $1 billion.
69
On June 9, 2021, each of Evana Yee Wah Hui and Paul J. Niewiadomski, and our former independent director accepted a letter agreement to act as our independent director and was awarded share options under the 2021 Share Option Plan (as defined below) to purchase 15,585 Ordinary Shares. The share options become effective upon the effectiveness of the registration statement for our IPO when the nominee’s directorship becomes effective. These options are exercisable at $9.50 per share, of which 25% vest on each anniversary over four years following the closing of our IPO and is valid for 10 years once vested.
On January 1, 2022, Dr. Lo accepted a letter agreement to act as our independent director and was awarded share options under the 2021 Share Option Plan to purchase 15,585 Ordinary Shares. These options are exercisable at $31.85 per share, of which 25% vest on each anniversary over four years following the closing of our IPO and is valid for 10 years once vested.
2021 Share Option Plan
We adopted a 2021 Share Option Plan (the “2021 Share Option Plan” or the “Plan”) on May 31, 2021. The Plan is a share-based compensation plan that provides for discretionary grants of share options to our key employees, directors and consultants. The purpose of the Plan is to recognize contributions made to our company and its subsidiaries by such individuals and to provide them with additional incentive to achieve our objectives. No grants have been made under the plan as of the date hereof. The following is a summary of the Plan and is qualified by the full text of the Plan.
Administration. The Plan will be administered by our CEO and Chairman of our board of directors.
Number of Ordinary Shares. The number of Ordinary Shares that may be issued under the Plan is the maximum aggregate number of Ordinary Shares reserved and available pursuant to this Plan shall be the aggregate of (i) 1,235,076 Ordinary Shares, and (ii) on each January 1, starting with January 1, 2022, an additional number of shares equal to the lesser of (A) 2% of the outstanding number of Ordinary Shares (on a fully-diluted basis) on the immediately preceding December 31, and (B) such lower number of Ordinary Shares as may be determined by our board of directors. If there is a forfeiture or termination without the delivery of Ordinary Shares or of other consideration of any option made under the Plan, the Ordinary Shares underlying such option, or the number of Ordinary Shares otherwise counted against the aggregate number of Ordinary Shares available under the Plan with respect to the option, to the extent of any such forfeiture or termination, shall again be, or shall become, available for granting options under the Plan. The number of Ordinary Shares issuable under the Plan is subject to adjustment, in the event of any reorganization, recapitalization, share split, share distribution, merger, consolidation, split-up, spin-off, combination, subdivision, consolidation or exchange of shares, any change in the capital structure of the company or any similar corporate transaction. As of the date of this Annual Report, a total of 1,250,661 Ordinary Shares were authorized for issuance under the Plan, options to purchase 1,250,661 Ordinary Shares have been granted, excluding, if any, awards that were forfeited or canceled after the relevant grant dates and awards that have been vested, and options to purchase 754,553 Ordinary Shares are outstanding.
Eligibility. All persons as the CEO and Chairman of our board of directors may select from among the employees, directors, and consultants of our company. The grant of options shall be approved by our board of directors.
Share Options. Our board of directors shall determine the provisions, terms, and conditions of each option including, but not limited to, the option vesting schedule, repurchase provisions, rights of first refusal, forfeiture provisions, form of payment (cash, shares, cashless settlement, or other consideration) upon settlement of the option, payment contingencies and the exercise price; each option will last for the term stated in the option agreement, provided, however that in the case of an option that is to qualify as an Incentive Share Option as such term is defined in Section 422 of the Code (as defined in the Plan), the term shall not exceed ten (10) years. It is intended that share options qualify as “performance-based compensation” under Section 162(m) of the Code and thus be fully deductible by us for federal income tax purposes, to the extent permitted by law.
70
Payment for Share Options and Withholding Taxes. Our board of directors may make one or more of the following methods available for payment of an option, including the exercise price of a share option, and for payment of the minimum required tax obligation associated with an award: (i) cash; (ii) cheque; (iii) with respect to options, payment through a broker-dealer sale and remittance procedure pursuant to which the optionee (A) shall provide written instructions to a Company designated brokerage firm to effect the immediate sale of some or all of the purchased Ordinary Shares and remit to us sufficient funds to cover the aggregate exercise price payable for the purchased Ordinary Shares and (B) shall provide written directives to us to deliver the certificates for the purchased Ordinary Shares directly to such brokerage firm in order to complete the sale transaction; (iv) cashless election; or (v) any combination of the foregoing methods of payment.
Upon exercise of an option, we shall have the right, but not the obligation (except as required by applicable law), to withhold or collect from optionee an amount sufficient to satisfy such tax obligations. The optionee will be solely responsible for his/her own tax obligations.
Amendment of Award Agreements; Amendment and Termination of the Plan; Term of the Plan. Our board of directors may at any time amend, suspend or terminate the Plan; provided, however, that no such amendment shall be made without the approval of our shareholders to the extent such approval is required by applicable laws, or if such amendment would adversely affect the right of any participant under any agreement in any material way without the written consent of the participant. No option may be granted during any suspension of the Plan or after termination of the Plan. No suspension or termination of the Plan shall adversely affect any rights under options already granted to an optionee. The Plan becomes effective upon the completion of our IPO on July 20, 2021. It shall continue in effect for a term of ten (10) years unless sooner terminated or unless renewed for another period not to exceed ten (10) years pursuant to shareholder approval.
On June 9, 2021, our board of directors granted 1,235,076 options to certain officers, directors and employees of our company under the 2021 Share Option Plan; provided that the grants of 46,755 options to the independent director nominees at that time will only become effective upon the effectiveness of the registration statement for our IPO when the nominee’s directorship becomes effective. These options will be exercisable at $9.50 per share, of which 25% vest on each anniversary over four years following the closing of our IPO and is valid for 10 years once vested.
On January 1, 2022, our board of directors granted 15,585 options to a director of our company, under the 2021 Share Option Plan. These options will be exercisable at $31.85 per share, of which 25% vest on each anniversary over four years following the closing of our IPO and is valid for 10 years once vested.
71
The following table summarizes, as of the date of this Annual Report, the number of Ordinary Shares underlying outstanding options granted to our directors and executive officers and to other individuals as a group under the 2021 Share Option Plan, excluding awards that were forfeited or cancelled after the relevant grant dates.
| Name | Ordinary Shares Underlying Outstanding Options Granted | Exercise Price ($ per Ordinary Share) | Date of Grant | Date of Expiration | ||||||||
| Executive officers as a group | 415,588 | $9.50 | June 2021 | Various dates from July 2032 to July 2035 | ||||||||
| Directors as a group | 46,755 | From $9.50 to $31.85 | Various dates from June 2021 to January 2022 | Various dates from July 2032 to January 2036 | ||||||||
| Other employees as a group | 292,210 | $9.50 | June 2021 | Various dates from July 2032 to July 2035 | ||||||||
In May 2022, all directors and employees who were previously granted stock options upon our IPO agreed to a further lock-up undertaking for a period of six months after their stock options become vested. As their stock options are set to vest on July 16, 2022, their shares has remained lock-up until January 16, 2024. Subsequently, all directors and employees agreed to further lock-up undertakings until January 20, 2025.
Employment Agreements
We have entered into written employment agreements with each of our executive officers. Each of our executive officers is employed for a fixed term subject to automatic renewal or a continuous term unless either we or the executive officer gives prior notice to terminate such employment. These agreements provide for notice periods of varying duration for termination of the agreement by us or by the relevant executive officer, during which time the executive officer will continue to receive salary and benefits. We may terminate the employment for cause, at any time, without notice or remuneration, for certain acts of the executive officer, such as conviction or plea of guilty to a felony or grossly negligent or dishonest acts to our detriment, or misconduct or a failure to perform agreed duties. Each executive officer has agreed to hold, both during and after the employment agreement expires, in strict confidence and not to use or disclose to any person, corporation or other entity without written consent, any confidential information, and has agreed to be bound by non-competition and non-solicitation restrictions set forth in their agreements. In addition, each executive officer has agreed to assign to us all his or her all inventions, improvements, designs, original works of authorship, formulas, processes, compositions of matter, computer software programs, databases, mask works and trade secrets.
C. Board Practices
Terms of Directors and Executive Officers
Our board of directors currently consists of five directors. Our directors may be elected by a resolution of our board of directors or by an ordinary resolution of our shareholders. Our directors are not subject to a term of office and hold office until the expiration of his or her term, as may be provided in a written agreement with our company, and his or her successor has been elected and qualified, until his or her resignation, until his or her office is otherwise vacated in accordance with our articles of association, or until such time as they are removed from office by ordinary resolution of the shareholders. If the director was appointed by our board of directors, such director holds office until the next following annual meeting of shareholders at which time such director is eligible for re-election. A director will cease to be a director if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; (ii) dies or is found by our company to be or becomes of unsound mind; (iii) resigned his or her office by notice in writing to our company; or (iv) without special leave of absence from our board of directors, is absent from three consecutive board meetings and our directors resolve that his office be vacated. There is no mandatory retirement age for directors. None of our directors has a service contract with us that provides for benefits upon termination of service, or an appropriate negative statement.
72
Our officers are elected by and serve at the discretion of our board of directors.
Duties of Directors
Under Cayman Islands law, all of our directors owe three types of duties to us: (i) statutory duties, (ii) fiduciary duties, and (iii) common law duties. The Companies Act (2020 Revision) of the Cayman Islands imposes a number of statutory duties on a director. A Cayman Islands director’s fiduciary duties are not codified, however, the courts of the Cayman Islands have held that a director owes the following fiduciary duties: (a) a duty to act in what the director bona fide considers to be in the best interests of the company, (b) a duty to exercise their powers for the purposes they were conferred, (c) a duty to avoid fettering his or her discretion in the future and (d) a duty to avoid conflicts of interest and of duty. The common law duties owed by a director are those to act with skill, care and diligence that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and, also, to act with the skill, care and diligence in keeping with a standard of care commensurate with any particular skill they have which enables them to meet a higher standard than a director without those skills. In fulfilling their duty of care to us, our directors must ensure compliance with our memorandum and articles of association, as amended from time to time. We have the right to seek damages if a duty owed by any of our directors is breached.
Our Amended and Restated Memorandum and Articles of Association ( “Amended Articles”) provide that a director must disclose the nature and extent of any material interests in any contract or arrangement, provided that the required notice has been given and subject to any separate requirement for Audit Committee approval under applicable law or the Nasdaq Stock Market listing rules, and unless disqualified by the chairman of the relevant board meeting, a director may vote in respect of any contract or arrangement in which such director is interested and may be counted in the quorum at such meeting. However, even if a director discloses his interest and is therefore permitted to vote, he must still comply with his duty to act bona fide in the best interest of our company.
Our directors also owe to us a duty to exercise the powers for the purpose for which they were given and the duty to act with skill and care. It was previously considered that a director need not exhibit in the performance of his or her duties a greater degree of skill than may reasonably be expected from a person of his or her knowledge and experience. However, courts are moving towards an objective standard with regard to the required skill and care and these authorities are likely to be followed in the Cayman Islands.
The functions and powers of our board of directors include, among others:
| ● | convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings; |
| ● | declaring dividends and distributions; |
| ● | appointing officers and determining the term of office of officers; |
| ● | exercising the borrowing powers of our company and mortgaging the property of our company; and |
| ● | approving the transfer of shares of our company, including the registering of such shares in our share register. |
Interested Transactions
Subject to our memorandum and articles of association, as amended from time to time, our director may vote, attend a board meeting or sign a document on our behalf with respect to any contract or transaction in which he or she is interested. A director must promptly disclose the interest to all other directors after becoming aware of the fact that he or she is interested in a transaction we have entered into or are to enter into. A general notice or disclosure to our board of directors or otherwise contained in the minutes of a meeting or a written resolution of the board of directors or any committee of the board of directors that a director is a shareholder, director, officer or trustee of any specified firm or company and is to be regarded as interested in any transaction with such firm or company will be sufficient disclosure, and, after such general notice, it will not be necessary to give special notice relating to any particular transaction. Any such transaction that would reasonably be likely to affect a director’s status as an “Independent Director”, or that would constitute a “related party transaction” as defined by Item 7.B of Form 20-F promulgated by the SEC, shall require the approval of the Audit Committee.
73
Remuneration and Borrowing
The directors may receive such remuneration as our board of directors may determine from time to time and in accordance with the recommendations of the compensation committee of our board of directors and our corporate governance documents. Each director is entitled to be repaid or prepaid all traveling, hotel and incidental expenses reasonably incurred or expected to be incurred in attending meetings of our board of directors or committees of our board of directors or shareholder meetings or otherwise in connection with the discharge of his or her duties as a director. The compensation committee will assist the directors in reviewing and approving the compensation structure for the directors. Our board of directors may exercise all the powers of our company to borrow money and to mortgage or charge our undertakings, property and assets both present and future an uncalled capital or any part thereof, and to issue debentures and other securities, whether outright or as collateral security for any of our debts, liabilities or obligations or any of our subsidiary undertaking or of any third party.
Qualification
There are no membership qualifications for directors. Further, there are no share ownership qualifications for directors unless so fixed by us in a general meeting by ordinary resolution of our shareholders. There are no other arrangements or understandings pursuant to which our directors are selected or nominated.
Committees of the Board of Directors
We have established an audit committee, a compensation committee and a nominating and corporate governance committee under our board of directors. We have adopted a charter for each of the three committees. Each of the committees of our board of directors shall have the composition and responsibilities described below.
Audit Committee
Evana Yee Wah Hui, Paul J. Niewiadomski and Dr. Wing Yan (William) Lo are the members of our Audit Committee, with Dr. Wing Yan (William) Lo serving as the chairperson. All members of our Audit Committee satisfy the independence requirements of Rule 5605(c)(2) of the Nasdaq Stock Market listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act.
We have adopted Audit Committee Charter on December 3, 2020 and the Audit Committee Charter became effective on July 15, 2021. In accordance with our Audit Committee Charter, our Audit Committee shall perform several functions, including:
| ● | evaluates the independence and performance of, and assesses the qualifications of, our independent auditor, and engages such independent auditor; |
| ● | approves the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services, and approves in advance any non-audit service to be provided by the independent auditor; |
| ● | monitors the independence of the independent auditor and the rotation of partners of the independent auditor on our engagement team as required by law; |
| ● | reviews the financial statements to be included in our annual reports on Form 20-F and quarterly reports on Form 6-K and reviews with management and the independent auditors the results of the annual audit and reviews of our quarterly financial statements; |
| ● | oversees all aspects our systems of internal accounting control and corporate governance functions on behalf of our board of directors; |
| ● | reviews and approves in advance any proposed related-party transactions and report to the full board of directors on any approved transactions; and |
| ● | provides oversight assistance in connection with legal, ethical and risk management compliance programs established by our management and our board of directors, including Sarbanes-Oxley Act implementation, and makes recommendations to our board of directors regarding corporate governance issues and policy decisions. |
Our board of directors has determined that Dr. Wing Yan (William) Lo possesses accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC.
74
Compensation Committee
Evana Yee Wah Hui, Paul J. Niewiadomski and Dr. Wing Yan (William) Lo are the members of our Compensation Committee with Evana Yee Wah Hui serving as the chairperson. All members of our Compensation Committee satisfy the independence requirements of Rule 5605(a)(2) of the Nasdaq Stock Market listing rules. We have adopted Compensation Committee Charter on December 3, 2020 and the Compensation Committee Charter became effective on July 15, 2021. In accordance with the Compensation Committee’s Charter, the Compensation Committee shall be responsible for overseeing and making recommendations to our board of directors regarding the salaries and other compensation of our executive officers and general employees and providing assistance and recommendations with respect to our compensation policies and practices, among other things.
Nominating and Corporate Governance Committee
Evana Yee Wah Hui, Paul J. Niewiadomski and Dr. Wing Yan (William) Lo are the members of our Nominating and Corporate Governance Committee with Paul J. Niewiadomski serving as the chairperson. All members of our Nominating and Corporate Governance Committee satisfy the independence requirements of Rule 5605(a)(2) of the Nasdaq Stock Market listing rules. We have adopted Nominating and Corporate Governance on December 3, 2020 and the Nominating and Corporate Governance Committee Charter became effective on July 15, 2021. In accordance with the Nominating and Corporate Governance Committee’s Charter, the Nominating and Corporate Governance Committee shall be responsible for identifying and proposing new potential director nominees to our board of directors for consideration and review our corporate governance policies, among other things.
Corporate Governance
As a Cayman Islands exempted company listed on the Nasdaq Stock Market, we are subject to the Nasdaq Stock Market’s corporate governance listing standards. However, Nasdaq rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards. We have followed on home country practice exemption with respect to the requirement of holding an annual general meeting of shareholders no later than one year after the end of its fiscal year-end. We may choose to follow additional home country practices in the future.
Our board of directors has adopted a code of business conduct and ethics that applies to our directors, principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, and employees. A copy of our code of business conduct and ethics is available on our website at https://www.regencellbioscience.com.
Director Independence
Our board of directors has reviewed the independence of our directors, applying the independence standards under the Nasdaq Stock Market listing rules. Based on this review, our board of directors determined that each of Evana Yee Wah Hui, Paul J. Niewiadomski and Dr. Wing Yan (William) Lo is “independent” within the meaning of the Nasdaq Stock Market listing rules. In making this determination, our board of directors considered the relationships that each of these non-employee directors has with us and all other facts and circumstances our board of directors deemed relevant in determining their independence. As required under applicable Nasdaq Stock Market listing rules, we anticipate that our independent directors will meet on a regular basis as often as necessary to fulfill their responsibilities, including at least annually in executive session without the presence of non-independent directors and management.
75
Board Diversity
The following board diversity matrix sets forth the information concerning the gender and demographic background of our board of directors, as self-identified by its members, in accordance with Nasdaq Stock Market listing rules.
| Country of Principal Executive Offices | Hong Kong | |
| Foreign Private Issuer | Yes | |
| Disclosure Prohibited Under Home Country Law | No | |
| Total Number of Directors | 5 |
| Female | Male | Non-Binary | Did Not Disclose Gender | ||||
| Part I: Gender Identity | |||||||
| Directors | 1 | 4 | 0 | 0 | |||
| Part II: Demographic Background | |||||||
| Underrepresented Individual in Home Country Jurisdiction | 0 | ||||||
| LGBTQ+ | 0 | ||||||
| Did Not Disclose Demographic Background | 0 | ||||||
D. Employees
As of June 30, 2024, 2023 and 2022, we had a total of 12, 12 and 13 full-time employees in Hong Kong. As of June 30, 2024, 4 of our full-time employees in Hong Kong were in research and development, 1 was in sales and marketing and 7 were in general administration.
We generally enter into standard confidentiality and employment agreements with our management and other employees. These contracts include a standard non-compete covenant that prohibits the employee from competing with us, directly or indirectly, during his or her employment and for certain years after the termination of his or her employment.
As required by law, our subsidiaries in Hong Kong make contributions equal to certain percentages of each qualifying employee’s salary to the Mandatory Provident Fund Scheme.
We believe that we maintain a good working relationship with our employees. We have not experienced any material labor disputes or work stoppages as of the date of this annual report. None of our employees are represented by labor unions or labor organizations or covered by a collective bargaining agreement.
E. Share Ownership
See Item 7 below.
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
76
Item 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table sets forth information with respect to beneficial ownership of our Ordinary Shares as of the date of this Annual Report by:
| ● | Each person who is known by us to beneficially own more than 5% of our outstanding Ordinary Shares; and |
| ● | Each of our director and executive officers. |
We have determined beneficial ownership in accordance with the rules of the SEC. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of the date of this Annual Report, including through the exercise of any options, warrants or other rights or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power or the power to receive the economic benefit with respect to all Ordinary Shares that they beneficially own, subject to applicable community property laws. None of the shareholders listed in the table are a broker-dealer or an affiliate of a broker dealer. Percentages of beneficial ownership are calculated based on 13,012,866 Ordinary Shares outstanding as of the date of this Annual Report. Unless otherwise indicated in the footnotes, the address for each beneficial owner is in the care of our company at 9/F Chinachem Leighton Plaza, 29 Leighton Road, Causeway Bay, Hong Kong.
| Ordinary Shares Beneficially Owned | Percentage of Votes Held | |||||||||||
| Number | Percent | Percent | ||||||||||
| Directors and Executive Officers: | ||||||||||||
| Yat-Gai Au (1) | 10,568,839 | 81.2 | %(1) | 81.2 | % | |||||||
| James Wai Hong Chung | - | - | - | |||||||||
| Yat-Pui Au | * | * | * | |||||||||
| Evana Yee Wah Hui | - | - | - | |||||||||
| Paul J. Niewiadomski | - | - | - | |||||||||
| Dr. Wing Yan (William) Lo | - | - | - | |||||||||
| All directors and executive officers as a group | 10,584,142 | 81.3 | %(1) | 81.3 | % | |||||||
| Principal Shareholders: | ||||||||||||
| Yat-Gai Au (1) | 10,568,839 | 81.2 | % | 81.2 | % | |||||||
| Digital Mobile Venture Ltd.(2) | 988,902 | 7.6 | % | 7.6 | % | |||||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | Represents Ordinary Shares held by Regencell (BVI) Limited, a British Virgin Islands company wholly-owned by Mr. Yat-Gai Au, our founder, director and CEO. Mr. Yat-Gai Au is deemed to be the beneficial owners of these securities. |
| (2) | Represents Ordinary Shares held by Digital Mobile Venture Ltd., a British Virgin Islands company, in which Samuel Chen and Fiona Chang are the directors and the stockholders according to the Schedule 13G filed by Digital Mobile Venture Ltd. on December 15, 2021. The business address of Digital Mobile Venture Ltd. is c/o Rayson Technology Co. Ltd., 5F, No. 550, Ruei Guang Road, Taipei, Taiwan. |
77
As of the date of this Annual Report, 2,670,761 of our Ordinary Shares were held by a record holder in the United States, which is Cede & Co, representing 20.5% of our total outstanding Ordinary Shares. The number of individual holders of record is based exclusively upon our share register and does not address whether a share or shares may be held by the holder of record on behalf of more than one person or institution who may be deemed to be the beneficial owner of a share or shares in our company. As a result, we may not be aware of each person or group of affiliated persons who beneficially own more than 5% of our Ordinary Shares.
To our knowledge, our company is not owned or controlled directly or indirectly by any government or by any corporation or by any other natural or legal person severally or jointly. We are not aware of any arrangements, known to us, the operation of which may at a subsequent date result in a change in control of our company. Our major shareholders do not have any special voting rights.
For certain information concerning the outstanding awards we have granted to our directors and executive officers individually pursuant to our 2021 Share Option Plan, see “Item 6. Directors, Senior Management and Employees – B. Compensation – 2021 Share Option Plan.” Other than under the 2021 Share Incentive Plan, there are no arrangements for involving the employees in the capital of our company, including any arrangement that involves the issue or grant of options or shares or securities of our company.
B. Related Party Transactions
The following is a description of transactions since beginning of our preceding three financial years up to the date hereof, in which the amount involved in the transaction exceeded or will exceed the lesser of $120,000 or one percent of the average of our total assets as at the year-end for the last three completed fiscal years, and to which any of our directors, executive officers or beneficial holders of more than 5% of our capital stock, or any immediate family member of, or person sharing the household with, any of these individuals, had or will have a direct or indirect material interest.
Rental Agreement with Ace United International Limited
On December 23, 2020, we entered into an office rental agreement with Ace United International Limited, which is a company wholly owned by our founder and CEO. The monthly rental is $4,103 and the terms of the agreement from the 1st of January to the 31st of December each year are renewed on an annual basis. The rental agreement was terminated in December 2021. The rental payment is expensed as incurred. During the years ended June 30, 2024, 2023 and 2022, the rental expenses were nil, nil and $24,615, respectively. The balance due to Ace United International Limited was nil as of June 30, 2024 and 2023.
Other Payables with Our Executive Management
As of June 30, 2024 and 2023, other payables – related parties represented reimbursement from related parties and the Company’s executive management, Mr. Chung and Mr. Au for out-of-pocket expenses incurred for business purposes. The balances due to Mr. Chung and Mr. Au were approximately $681 and $7,606, respectively, as of June 30, 2024 and $1,476 and $5,512, respectively, as of June 30, 2023.
During the years ended June 30, 2024 and 2023, the expenses paid for TCM formulae and materials for product development to Regeneration Company Limited, a related company, were approximately $0.09 million and $0.6 million, respectively. The balance due to Regeneration Company Limited was approximately $156 and $1,506 respectively as of June 30, 2024 and 2023.
During the year ended June 30, 2024 and 2023, the expenses paid for services fee for research support to Regencell Bioscience Asia Sdn. Bhd., a related company, were approximately $0.03 million and $0.03 million, respectively. The balance due to Regencell Bioscience Asia Sdn. Bhd., was nil and $2,076 as of June 30, 2024 and 2023.
78
2021 Share Option Plan
See “Item 6. Directors, Senior Management and Employees – B. Compensation – 2021 Share Option Plan.”
Employment Agreements
See “Item 6. Directors, Senior Management and Employees – B. Compensation - Employment Agreements”
Partnership Agreements with TCM Practitioner
In January 2018, we entered into a Strategic Partnership Agreement with the TCM Practitioner, the father of our CEO and director. Pursuant to the Strategic Partnership Agreement we have with TCM Practitioner, we have exclusive rights and ownership of all his TCM formulae for patients suffering from ADHD and ASD, and all other TCM formulae targeting different kinds of human illnesses, disorders and degeneration as well as the intellectual property rights of the TCM formulae, including research and development, trademark, copyright, patent, and any other intellectual property rights in relation to the TCM formulae that he owns. The TCM Practitioner is authorized to conduct research studies on these TCM formulae. Any inventions, TCM formulae, utilities, models’ improvements, research, discoveries, designs, processes, methods of manufacture, and products conceived or made by the TCM Practitioner in relation to TCM shall be the sole and exclusive property of us.
The Strategic Partnership Agreement does not have a termination date and will remain effective indefinitely under Hong Kong law. The Strategic Partnership Agreement can be amended or terminated by the mutual written agreement of the parties without breach. Pursuant to the Strategic Partnership Agreement, in exchange for the rights, we are required to donate three percent (3.0%) of net revenue as shown in the audited financial accounts that we generate in association with the use and/or commercialization of the TCM formulae to any of charitable institutions and/or trusts of a public character anywhere in the world at the sole and absolute choice of the TCM Practitioner and in such proportion at the sole and absolute discretion of the TCM practitioner on a yearly basis. We also undertake to pay for all reasonable costs and expenses incurred by the TCM Practitioner in conducting research, testing, attending meetings/seminars, compiling records, or performing any similar acts in relation to the development of the TCM formulae.
Supplemental Agreement with TCM Practitioner
In November 2020, we entered into a Supplemental Agreement with the TCM Practitioner. Under the Supplemental Agreement, the TCM Practitioner shall provide his research by using his best endeavors on his TCM formulae and TCM inventions under our direction and supervision. We shall pay the TCM Practitioner for his expenses on his TCM research within 30 days upon the receipt of invoice. We have authorized the TCM Practitioner, his agents, subcontractors, development team, and affiliates to use TCM formulae and TCM inventions to conduct research. However, the authorized parties, including TCM Practitioner, shall not directly or indirectly publish, disseminate or otherwise disclose, deliver or make available to any third party any confidential information, without our prior written consent and notice. The confidential information includes all information, know-how, and records in any way connected with our company and our research, including all TCM inventions, TCM formulae, and intellectual property rights, data, operations and testing procedures, and all patients and supplier information, etc. The confidentiality obligations shall survive notwithstanding the termination of the Supplemental Agreement for ten (10) years thereafter. The TCM Practitioner also shall not to be directly or indirectly concerned with or engaged or interested in any other business which is in any respect in competition with or similar to the TCM business conducted by our company for two (2) years after the expiration or termination of the Supplemental Agreement.
The Supplemental Agreement shall remain effective until the Strategic Partnership Agreement is expired or terminated. We may terminate this Supplemental Agreement for any reason in our sole discretion and without any indemnity or damages being due to TCM Practitioner with thirty (30) days prior written notice. The TCM Practitioner may terminate this Supplemental Agreement in the event that we fail to meet our payment obligations under Supplemental Agreement which is not cured within thirty (30) days of the notification of such by us.
79
Policies and Procedures for Related Party Transactions
Our board of directors has established an audit committee which is responsible for reviewing and approving all related party transactions.
C. Interests of Experts and Counsel
Not applicable.
Item 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this Annual Report.
Legal Proceedings
From time to time, we may be subject to legal or administrative proceedings, investigations and claims arising in the ordinary course of our business. We are not currently a party to any legal proceeding, investigation or claim, or any pending legal proceeding, investigation or claim, which, in the opinion of our management, is likely to have a material adverse effect on our business, financial condition or results of operations, nor are we aware of any such proceedings, investigation or claim threatened against us or our subsidiaries.
Dividend Policy
We intend to keep any future earnings to finance the expansion of our business, and we do not anticipate that any cash dividends will be paid in the foreseeable future.
Our board of directors has discretion as to whether to distribute dividends, subject to certain requirements of Cayman Islands law. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our directors. Under Cayman Islands law, a Cayman Islands exempted company may pay a dividend on its shares out of either distributable profits or amounts standing to the credit of the share premium amount account, provided that in no circumstances may a dividend be paid if this would result in the company being unable to pay its debts due in the ordinary course of business.
If we determine to pay dividends on any of our Ordinary Shares in the future, as a holding company, we will be dependent on additional debt or equity financing by the holding company and/or receipt of funds from our subsidiaries, Regencell Bioscience Limited and Regencell Limited, which are incorporated in Hong Kong. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors may deem relevant.
B. Significant Changes
Except as disclosed elsewhere in this Annual Report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this Annual Report.
Item 9. THE OFFER AND LISTING
A. Offering and Listing Details.
Our Ordinary Shares are currently listed on Nasdaq Capital Market under the symbol “RGC.”
B. Plan of Distribution
Not applicable.
80
C. Markets
Our Ordinary Shares are currently listed on Nasdaq Capital Market under the symbol “RGC”.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
Item 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
The information required by Item 10.B of Form 20-F is included in the section titled “Description of Share Capital” in our IPO prospectus included in our registration statement on Form F-1 (File No. 333-254571), which section is incorporated herein by reference. A copy of our Amended and Restated Memorandum and Articles of Association adopted on May 31, 2021 was filed as Exhibit 3.2 to Amendment No. 2 to our registration statement on Form F-1 (File No. 333-254571), which was filed with the SEC on June 11, 2021, and is hereby incorporated by reference into this Annual Report.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in this Annual Report.
D. Exchange Controls
Under Cayman Islands law, there are currently no restrictions on the export or import of capital, including foreign exchange controls or restrictions that affect the remittance of dividends, interest or other payments to nonresident holders of our shares.
E. Taxation
The following summary of the material Cayman Islands, Hong Kong and U.S. federal income tax consequences of an investment in our Ordinary Shares is based upon laws and relevant interpretations thereof in effect as of the date of this Annual Report, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in our Ordinary Shares, such as the tax consequences under state, local and other tax laws.
81
Cayman Islands Taxation
The Cayman Islands currently levy no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to our company levied by the Government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or after execution brought within the jurisdiction of the Cayman Islands. No stamp duty is payable in the Cayman Islands on the issue of shares by, or any transfers of shares of, Cayman Islands companies (except those which hold interests in land in the Cayman Islands). The Cayman Islands is not party to any double tax treaties that are applicable to any payments made to or by our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of our Ordinary Shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of our Ordinary Shares, as the case may be, nor will gains derived from the disposal of our Ordinary Shares be subject to Cayman Islands income or corporation tax.
Hong Kong Taxation
The following summary of certain relevant taxation provisions under the laws of Hong Kong is based on current law and practice and is subject to changes therein. This summary does not purport to address all possible tax consequences relating to purchasing, holding or selling our Ordinary Shares, and does not take into account the specific circumstances of any particular investors, some of whom may be subject to special rules. Accordingly, holders or prospective purchasers (particularly those subject to special tax rules, such as banks, dealers, insurance companies and tax-exempt entities) should consult their own tax advisors regarding the tax consequences of purchasing, holding or selling our Ordinary Shares. Under the current laws of Hong Kong:
| ● | No profit tax is imposed in Hong Kong in respect of capital gains from the sale of our Ordinary Shares. |
| ● | Revenue gains from the sale of our Ordinary Shares by persons carrying on a trade, profession or business in Hong Kong where the gains are derived from or arise in Hong Kong from the trade, profession or business will be chargeable to Hong Kong profits tax, which is currently imposed at the rate of 16.5% on corporations and at a maximum rate of 15% on individuals and unincorporated businesses. |
| ● | Gains arising from the sale of our Ordinary Shares, where the purchases and sales of our Ordinary Shares are effected outside of Hong Kong such as, for example, on the Nasdaq, should not be subject to Hong Kong profits tax. |
According to the current tax practice of the Hong Kong Inland Revenue Department, dividends paid on our Ordinary Shares would not be subject to any Hong Kong tax.
No Hong Kong stamp duty is payable on the purchase and sale of our Ordinary Shares.
Material U.S. Federal Income Tax Considerations
The following is a summary of certain material U.S. federal income tax consequences to U.S. Holders (defined below) of the ownership and disposition of our Ordinary Shares. For purposes of this summary, a “U.S. Holder” means a beneficial owner of our Ordinary Shares that is for U.S. federal income tax purposes:
| ● | an individual citizen or resident of the United States; | |
| ● | a corporation (or other entity classified as a corporation for U.S. federal income tax purposes) that is created or organized in or under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or | |
| ● | a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more United States persons (as defined in Section 7701(a)(30) of the Code (defined below)) are authorized to control all substantial decisions of the trust, or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a United States person. |
82
This summary is based on the Internal Revenue Code of 1986, as amended (the “Code”), as in effect on the date of this Annual Report and on U.S. Treasury regulations in effect or, in some cases, proposed, as of the date of this annual report, as well as judicial and administrative interpretations thereof available on or before such date. These authorities are subject to change or differing interpretations, possibly on a retroactive basis.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a U.S. Holder based on such U.S. Holder’s individual circumstances. In particular, this discussion considers only U.S. Holders that own our Ordinary Shares as capital assets within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion also does not address the potential application of the alternative minimum tax, the Medicare contribution tax on certain net investment income, or the U.S. federal income tax consequences to U.S. Holders that are subject to special rules, including:
| ● | financial institutions or financial services entities; | |
| ● | brokers, dealers or traders in securities, commodities or currencies; | |
| ● | persons who have elected mark-to-market accounting; |
| ● | tax-exempt entities, “individual retirement accounts” or “Roth IRAs”; | |
| ● | governments or agencies or instrumentalities thereof; | |
| ● | insurance companies; | |
| ● | regulated investment companies; | |
| ● | real estate investment trusts; |
| ● | certain expatriates or former long-term residents of the United States; | |
| ● | persons that actually or constructively own 5% or more, by voting power or value, of our outstanding Ordinary Shares; | |
| ● | persons that acquired our Ordinary Shares pursuant to the exercise of employee stock options, in connection with employee stock incentive plans or otherwise as compensation; | |
| ● | persons that will hold our Ordinary Shares in connection with a trade or business outside the United States; | |
| ● | persons that hold our Ordinary Shares as part of a straddle, constructive sale, hedging, conversion or other integrated transaction; or | |
| ● | persons whose functional currency is not the U.S. dollar. |
This discussion does not address any aspect of U.S. federal non-income tax laws, such as gift or estate tax laws, or state, local or non-U.S. tax laws. Additionally, this discussion does not consider the tax treatment of entities or arrangements classified as partnerships for U.S. federal income tax purposes or other pass-through entities or persons who hold our Ordinary Shares through such entities or arrangements. If a partnership (or other entity or arrangement classified as a partnership for U.S. federal income tax purposes) is the beneficial owner of our Ordinary Shares, the U.S. federal income tax treatment of a partner in the partnership will generally depend on the status of the partner and the activities of the partnership. This discussion also assumes that any distribution made (or deemed made) in respect of our Ordinary Shares and any consideration received (or deemed received) by a U.S. Holder in connection with the sale or other disposition of such shares will be in U.S. dollars.
83
We have not sought, and will not seek, a ruling from the U.S. Internal Revenue Service (or “IRS”), or an opinion of counsel as to any U.S. federal income tax consequence described herein. The IRS may disagree with one or more aspects of the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
BECAUSE OF THE COMPLEXITY OF THE TAX LAWS AND BECAUSE THE TAX CONSEQUENCES TO ANY PARTICULAR HOLDER OF OUR ORDINARY SHARES MAY BE AFFECTED BY MATTERS NOT DISCUSSED HEREIN, EACH HOLDER OF OUR ORDINARY SHARES IS URGED TO CONSULT WITH ITS TAX ADVISOR WITH RESPECT TO THE SPECIFIC TAX CONSEQUENCES OF THE OWNERSHIP AND DISPOSITION OF OUR ORDINARY SHARES, INCLUDING THE APPLICABILITY AND EFFECT OF STATE, LOCAL AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS AND ANY APPLICABLE TAX TREATIES.
Passive Foreign Investment Company
A non-U.S. corporation will be classified as a PFIC for U.S. federal income tax purposes for any taxable year in which, after applying certain look-through rules, either:
| ● | at least 75% of its gross income is passive income; or | |
| ● | at least 50% of its assets (generally based on an average of the quarterly values of the assets during a taxable year) is attributable to assets that produce or are held for the production of passive income. |
For purposes of the above calculations, a non-U.S. corporation will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of any other corporation in which it owns, directly or indirectly, 25% or more (by value) of the equity. Passive income generally includes dividends, interest, certain rents or royalties, foreign currency or other investment gains and certain other categories of income. We must make a separate determination each taxable year as to whether we are a PFIC.
Based on the value of our assets for our taxable year ended June 30, 2024, including the value of our goodwill, and the composition of our income and assets in such taxable year, we believe we were a PFIC for our taxable year ended June 30, 2024. Specifically, we believe we were a PFIC for such taxable year due to the amount of interest income we received relative to other income we received during such taxable year. The application of the PFIC rules is subject to uncertainty in several respects. The classification of certain of our income as active or passive, and certain of our assets as producing active or passive income, and hence whether we may be or may become a PFIC, depends on the interpretation of certain U.S. Treasury regulations as well as certain IRS guidance relating to the classification of assets as producing active or passive income. Such regulations and guidance are potentially subject to different interpretations. Until we become revenue-generating, our PFIC status may depend, in part, on the receipt and treatment of passive income from interest-bearing accounts or other investments relative to other sources of income (including government grants).
Furthermore, for purposes of the asset test described above, goodwill is generally characterized as an active asset to the extent it is associated with business activities that produce active income, and the value of our assets, including goodwill, generally will be calculated using the market price of our Ordinary Shares, which may fluctuate considerably, especially in times of high market volatility. Accordingly, fluctuations in the market price of our Ordinary Shares may affect our PFIC status for any taxable year. In addition, cash is generally characterized as a passive asset for these purposes, so the composition of our income and assets will be affected by how, and how quickly, we spend the cash that we hold. Accordingly, we cannot assure you that we will not be a PFIC for the taxable year ending June 30, 2025 or any other future taxable year. We are under no obligation to take steps to reduce the risk of our being classified as a PFIC, and as stated above, the determination of the value of our assets will depend upon material facts (including the market price of our Ordinary Shares from time to time) that may not be within our control.
84
If we are a PFIC for any taxable year during which you hold Ordinary Shares, we will generally continue to be treated as a PFIC with respect to you for all succeeding taxable years during which you hold Ordinary Shares. If we cease to be a PFIC and you did not previously make a timely “mark-to-market” election as described below, however, you may avoid some of the adverse effects of the PFIC regime by making a “purging election” (as described below) with respect to the Ordinary Shares.
If we are a PFIC for any taxable year during which you hold Ordinary Shares, you will generally be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you realize from a sale or other disposition (including a pledge) of the Ordinary Shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the Ordinary Shares will be treated as an excess distribution. Under these special tax rules:
| ● | the excess distribution or gain will be allocated ratably over your holding period for the Ordinary Shares; |
| ● | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and |
| ● | the amount allocated to each other taxable year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to taxable years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the Ordinary Shares cannot be treated as capital, even if you hold the Ordinary Shares as capital assets.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the tax treatment discussed above. If you make a mark-to-market election for the Ordinary Shares, you will include in income each taxable year an amount equal to the excess, if any, of the fair market value of the Ordinary Shares as of the close of your taxable year over your adjusted basis in such Ordinary Shares. You are allowed a deduction for the excess, if any, of the adjusted basis of the Ordinary Shares over their fair market value as of the close of the taxable year. However, deductions are allowable only to the extent of any net mark-to-market gains on the Ordinary Shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of the Ordinary Shares, are treated as ordinary income. Ordinary loss treatment also applies to the deductible portion of any mark-to-market loss on the Ordinary Shares, as well as to any loss realized on the actual sale or disposition of the Ordinary Shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such Ordinary Shares. Your basis in the Ordinary Shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions as discussed below under “—Taxation of Distributions Paid on Ordinary Shares” would generally apply, except that the lower applicable capital gains rate for qualified dividend income discussed therein generally would not apply.
The mark-to-market election is available only for “marketable stock,” which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market (as defined in applicable U.S. Treasury regulations), including the Nasdaq Capital Market. If the Ordinary Shares are regularly traded on the Nasdaq Capital Market and if you are a holder of Ordinary Shares, the mark-to-market election would generally be available to you if we are or become a PFIC.
Alternatively, a U.S. Holder of stock in a PFIC may, at taxpayer’s discretion, make a “qualified electing fund” election with respect to such PFIC to elect out of the tax treatment discussed above. A U.S. Holder who makes a valid qualified electing fund election with respect to a PFIC will generally include in gross income for a taxable year such U.S. Holder’s pro rata share of the corporation’s earnings and profits for the taxable year. However, the qualified electing fund election is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. We do not currently intend to prepare or provide the information that would enable you to make a qualified electing fund election. If you hold Ordinary Shares in any year in which we are a PFIC, you will generally be required to file IRS Form 8621 regarding distributions received on the Ordinary Shares and any gain realized on the disposition of the Ordinary Shares.
85
If you do not make a timely “mark-to-market” election (as described above), and if we are a PFIC at any time during the period you hold our Ordinary Shares, then such Ordinary Shares will continue to be treated as stock of a PFIC with respect to you even if we cease to be a PFIC in a future taxable year, unless you make a “purging election” for the year we cease to be a PFIC. A “purging election” creates a deemed sale of such Ordinary Shares at their fair market value on the last day of the last taxable year in which we are treated as a PFIC. The gain recognized by the purging election will be subject to the special tax and interest charge rules treating the gain as an excess distribution, as described above. As a result of the purging election, you will have a new basis (equal to the fair market value of the Ordinary Shares on the last day of the last taxable year in which we are treated as a PFIC) and holding period (which new holding period will begin the day after such last day) in your Ordinary Shares for U.S. federal income tax purposes.
If we are treated as a PFIC with respect to you for any taxable year, to the extent any of our subsidiaries are also PFICs (“lower-tier PFICs”), you may be deemed to own shares in such lower-tier PFICs that are directly or indirectly owned by us in that proportion which the value of the Ordinary Shares you own bears to the value of all of our Ordinary Shares, and you may be subject to the adverse tax consequences described above with respect to the shares of such lower-tier PFICs that you would be deemed to own. However, an election for mark-to-market treatment would likely not be available with respect to any such lower-tier PFICs. You should consult your tax advisors regarding the availability and desirability of a mark-to-market election as well as the impact of such election on interests in any lower-tier PFICs.
If we are considered a PFIC, a U.S. Holder will also be subject to information reporting requirements on an annual basis. If we are or become a PFIC, you should consult your tax advisor regarding any reporting requirements that may apply to you.
U.S. HOLDERS ARE STRONGLY URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE IMPACT OF OUR BEING A PFIC FOR OUR TAXABLE YEAR ENDED JUNE 30, 2024 AND WITH RESPECT TO THE OPERATION OF THE PFIC RULES AND RELATED REPORTING REQUIREMENTS IN LIGHT OF THEIR PARTICULAR CIRCUMSTANCES, INCLUDING THE ADVISABILITY AND EFFECTS OF MAKING ANY ELECTION THAT MAY BE AVAILABLE.
Taxation of Distributions Paid on Ordinary Shares
Subject to the PFIC rules discussed above, the gross amount of distributions made by us to you with respect to the Ordinary Shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your Ordinary Shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. However, we do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will generally be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the Ordinary Shares are readily tradable on an established securities market in the United States (including the NASDAQ Capital Market), or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a PFIC for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Because there is no income tax treaty between the United States and the Cayman Islands, it is expected that clause (1) above can be satisfied only if the Ordinary Shares are readily tradable on an established securities market in the United States. You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our Ordinary Shares, including the effects of any change in law after the date of this Annual Report.
86
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend considered for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our Ordinary Shares will generally constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.” A U.S. Holder that does not elect to claim a foreign tax credit with respect to any foreign taxes for a given taxable year may instead claim an itemized deduction for all foreign taxes paid or accrued in that taxable year. The rules governing the U.S. foreign tax credit are complex, and you should consult your tax advisors to determine whether and to what extent a credit would be available in your particular circumstances, including the effects of any applicable income tax treaty.
Taxation of Dispositions of Ordinary Shares
Subject to the PFIC rules discussed above, you will generally recognize taxable gain or loss on any sale, exchange or other taxable disposition of an Ordinary Share equal to the difference between the amount realized (in U.S. dollars) for the Ordinary Share and your adjusted tax basis (in U.S. dollars) in the Ordinary Share. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the Ordinary Shares for more than one year, you will generally be eligible for reduced tax rates. The deductibility of capital losses may be subject to limitations. Any such gain or loss that you recognize will generally be treated as United States source income or loss for foreign tax credit limitation purposes which will generally limit the availability of foreign tax credits. The rules governing the U.S. foreign tax credit are complex, and you should consult your tax advisors to determine whether and to what extent a credit would be available in your particular circumstances, including the effects of any applicable income tax treaty.
Backup Withholding Tax and Information Reporting Requirements
Dividends on and the proceeds of a sale or other taxable disposition of Ordinary Shares may be subject to information reporting to the IRS and possible U.S. backup withholding. Backup withholding will not apply to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification or who is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status can provide such certification on IRS Form W-9. U.S. Holders should consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability, and a U.S. Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Additional Reporting Requirements
Individuals (and certain entities) that own “specified foreign financial assets” with an aggregate value in excess of certain thresholds on the last day of the taxable year (or with an aggregate value in excess of certain thresholds at any time during the taxable year) are generally required to file an information report on IRS Form 8938 with respect to such assets with their U.S. federal income tax returns. “Specified foreign financial assets” include any financial accounts maintained by foreign financial institutions, as well as any of the following, but only if they are not held in accounts maintained by certain financial institutions: (1) stocks and securities issued by non-U.S. persons, (2) financial instruments and contracts held for investment that have non-U.S. issuers or counterparties, and (3) interests in foreign entities. The Ordinary Shares may be subject to these rules. U.S. Holders are urged to consult their tax advisors regarding the application of these rules to their ownership of the Ordinary Shares.
87
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We previously filed with the SEC our registration statement on Form F-1 (File Number 333-254571), as amended, and a prospectus under the Securities Act with respect to our Ordinary Shares. We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F within four months after the end of each fiscal year. We have filed this Annual Report on Form 20-F, including exhibits, and furnished other current reports, with the SEC. As allowed by the SEC, in Item 19 of this Annual Report, we incorporate by reference certain information we filed with the SEC. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this Annual Report.
You may read and copy this Annual Report, including the exhibits incorporated by reference in this Annual Report, and our reports and other information, when so filed, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549 and at the SEC’s regional offices in New York, New York and Chicago, Illinois. You also can request copies of this Annual Report, including the exhibits incorporated by reference in this Annual Report, and our reports and other information, upon payment of a duplicating fee, by writing information on the operation of the SEC’s Public Reference Room.
The SEC also maintains a website at www.sec.gov that contains reports, proxy statements and other information regarding registrants that are filed electronically with the SEC. Our annual reports and some of the other information filed by us with the SEC may be accessed through this web site.
As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
Our financial statements have been prepared in accordance with U.S. GAAP.
We will furnish our shareholders with annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
88
Item 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
For purposes of this Item 11, reference to the “Group” means Regencell Bioscience Holdings Limited and all of its subsidiaries.
Foreign Exchange Risk
Currency risk is the risk that the value of financial assets or liabilities will fluctuate due to changes in foreign exchange rates.
As of June 30, 2024, 2023 and 2022, the Group has no significant foreign currency risk because most of the transactions are denominated in Hong Kong dollar or the United States dollar. Since Hong Kong dollar is pegged to the United States dollar, the Group’s exposure to foreign currency risk in respect of the balances denominated in Hong Kong dollars is considered to be minimal.
Credit Risk
Financial assets which potentially subject the Group to concentrations of credit risk consist principally of bank deposits and balances.
The Group limits its exposure to credit risk by transacting all of its securities and contractual commitment activities with broker-dealers, banks and regulated exchanges with high credit ratings and that the Group considers to be well established.
Liquidity Risk
Liquidity risk is the risk that the Group will encounter difficulty in raising funds to meet commitments associated with financial assets and liabilities. Liquidity risk may result from an inability to sell a financial asset quickly at an amount close to its fair value. The Company’s strategy is to minimize its exposure to liquidity risk by monitoring its liquid capital from time to time. In managing its liquidity risk, the Company monitors and maintains a level of cash and bank balances deemed adequate by management to finance the Company’s operations.
Interest Rate Risk
Interest rate risk arises from the possibility that changes in interest rates will affect future cash flows or the fair values of financial instruments.
The Group’s cash held with the banks are exposed to interest rate risk. However, our management considers the risk to be minimal as they are short-term with terms less than one month.
Inflation Risk
In recent years, inflation has not had a material impact on our results of operations.
Item 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
89
Part II
Item 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
Item 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
See “Item 10. Additional Information” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the Registration Statement on Form F-1, as amended (File Number: 333-254571) which was declared effective by the SEC on July 15, 2021 and was in relation to our IPO of 2,300,000 Ordinary Shares, and 325,000 Ordinary Shares sold in the over-allotment in the IPO, at the offering price of $9.50 per Ordinary Share. Our IPO closed in July 2021. Maxim Group LLC is the representative of the underwriter and sole book-running manager for our IPO. Gross proceeds from our IPO, including proceeds from the exercise of the over-allotment option, totaled approximately $24.93 million, before deducting underwriting discounts and other related expenses. We have also agreed to issue to the representative of the underwriter for our IPO warrants to purchase 65,625 Ordinary Shares of our company.
From the effective date of the registration statement on Form F-1 to June 30, 2024, the ending date of the reporting period of this Annual Report, our expenses incurred and paid to others in connection with the issuance and distribution of our Ordinary Shares in our IPO totaled $2.26 million, which included $1.83 million for underwriting discounts and commissions and $0.43 million for other expenses. None of the transaction expenses included any direct or indirect payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates.
We received net proceeds of approximately $22.67 million from our IPO, including the exercise of over-allotment option, after deducting these total expenses.
From the effective date of the registration statement on Form F-1 to June 30, 2024, the ending date of the reporting period of this Annual Report, we used approximately $5.4 million for staff salaries; approximately $1.6 million for general and administrative expenses, marketing, research and development and potential expansion of new businesses, approximately $3.3 million for facilities rental, renovations and equipment; approximately $1.5 million for second research study; approximately $0.02 million for product and intellectual properties registration; approximately $2.5 million for working capital and other general corporate purposes; and approximate $0.4 million to repay our shareholder loan with Mr. Yat-Gai Au. Other than as disclosed above, none of the net proceeds from our IPO were paid, directly or indirectly, to any of our directors, officers or their associates, persons owning 10% or more of our equity securities or our affiliates.
As of June 30, 2024, $8.0 million of the net proceeds from our IPO remained unused. We have placed the remaining net proceeds from our IPO in our savings account and short-term investment. We still intend to continue to use the net proceeds from our IPO as disclosed in our registration statements on Form F-1.
Item 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and our financial controller, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report, as required by Rule 13a-15(b) under the Exchange Act. Based on that evaluation, our management has concluded that our disclosure controls and procedures were not effective as of June 30, 2024 because of the material weaknesses in our internal control over financial reporting identified, as described in “Management’s Annual Report on Internal Control over Financial Reporting” below.
90
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15 (f) under the Exchange Act, for our company. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with generally accepted accounting principles and includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets, (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are being made only in accordance with authorizations of a company’s management and directors, and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the consolidated financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As required by Section 404 of the Sarbanes-Oxley Act and related rules as promulgated by the SEC, our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2024 using criteria established in “Internal Control—Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on this assessment, our management has concluded that our internal control over financial reporting was ineffective as of June 30, 2024, due to the material weaknesses in our internal control over financial reporting identified.
In connection with the preparation and audit of our consolidated financial statements as of and for the year ended June 30, 2024, our management identified the following three material weaknesses in our internal control over financial reporting: (1) a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements; (2) our lack of internal audit function to establish formal risk assessment process and internal control framework; and (3) our lack of segregation of duties for certain key functions due to limited staff and resources.
As defined under standards established by PCAOB, a material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
As a result, we have already taken some steps and have continued to implement measures to remediate the material weaknesses identified, including but not limited to (i) continuing to recruit experienced personnel with relevant past experience working on U.S. GAAP and SEC reporting, (ii) improving the monitoring of and oversight controls for non-recurring transactions to ensure the accuracy and completeness of financial reporting and (iii) engaging external experts to assist with should there be non-recurring and complex transactions and continuing to implement measures to remediate our internal control deficiencies.
We are fully committed to continue to implement measures to remediate our material weaknesses, significant deficiencies and other control deficiencies in our internal control over financial reporting. However, the implementation of these measures may not fully address the material weaknesses in our internal control over financial reporting. We are not able to estimate with reasonable certainty the costs that we will need to incur to implement these and other measures designed to improve our internal control over financial reporting.
The process of designing and implementing an effective financial reporting system is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a financial reporting system that is adequate to satisfy our reporting obligations. See “Item 3. Key Information—D. Risk Factors—General Company Related Risks —We have identified material weaknesses in our internal control over financial reporting. If we fail to develop and maintain an effective system of internal control over financial reporting, we may be unable to accurately report our financial results or prevent fraud.”
91
Attestation Report of the Registered Public Accounting Firm
Since we qualified as an “emerging growth company” as defined under the JOBS Act as of June 30, 2024, we are exempt from the requirement to comply with the auditor attestation requirements that our independent registered public accounting firm attest to and report on the effectiveness of our internal control structure and procedures for financial reporting. This Annual Report on Form 20-F does not include an attestation report of our independent registered public accounting firm due to a transition period established by rules of the SEC for emerging growth companies.
Changes in Internal Control over Financial Reporting
Other than those disclosed above, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the period covered by this Annual Report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Our board of directors has determined that Dr. Wing Yan (William) Lo, an independent director and the chairman of our Audit Committee, qualifies as an “audit committee financial expert” within the meaning of the SEC rules and possesses financial sophistication within the meaning of the Nasdaq Stock Market listing rules. Dr. Wing Yan (William) Lo satisfies the independence requirements of Rule 5605(c)(2) of the Nasdaq Stock Market listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act.
Item 16B. CODE OF ETHICS
Our board of directors has adopted a code of business conduct and ethics that applies to our directors, principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, and employees. A copy of our code of business conduct and ethics is available on our website at https://www.regencellbioscience.com.
Item 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The Company was notified by Friedman LLP, the Company’s former independent registered public accounting firm, that effective September 1, 2022, Friedman LLP merged with Marcum LLP and continued to operate as an independent registered public accounting firm. Friedman LLP continued to serve as the Company’s independent registered public accounting firm through December 31, 2022. On February 22, 2023, the audit committee of the board of directors of the Company approved the engagement of Marcum Asia CPAs LLP (“Marcum Asia”) to serve as the independent registered public accounting firm of the Company. The services previously provided by Friedman LLP are now provided by Marcum Asia.
The following table represents the approximate aggregate fees for services rendered by Friedman LLP and Marcum Asia, our independent registered public accounting firm, for the periods indicated:
For the Years Ended June 30, | ||||||||
| 2024 | 2023 | |||||||
| Marcum Asia | ||||||||
| Audit Fees | $ | 120,000 | $ | 120,000 | ||||
| Audit Related Fees | 30,000 | - | ||||||
| Friedman LLP | ||||||||
| Audit Fees | - | - | ||||||
| Audit Related Fees | - | 30,000 | ||||||
| Total Fees | $ | 150,000 | $ | 150,000 | ||||
92
“Audit-related fees” are the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit and are not reported under audit fees. These fees primarily include interim review fee.
The policy of our audit committee is to pre-approve all audit and non-audit services provided by our independent auditor including audit services, audit-related services, tax services and other services.
Our Audit Committee evaluated and approved in advance the scope and cost of the engagement of an auditor before the auditor rendered its audit and non-audit services.
Item 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
Item 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
Item 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
The Company was notified by Friedman LLP, the Company’s then independent registered public accounting firm, that effective from September 1, 2022, Friedman LLP merged with Marcum LLP and continued to operate as an independent registered public accounting firm. Friedman LLP continued to serve as the Company’s independent registered public accounting firm through December 31, 2022. On February 16, 2023, the Company engaged Marcum Asia to serve as the independent registered public accounting firm of the Company. On February 22, 2023, the audit committee of the board of directors of the Company approved the engagement of Marcum Asia to serve as the independent registered public accounting firm of the Company. The services previously provided by Friedman LLP are now provided by Marcum Asia.
See the current report on Form 6-K (File No. 001-40617) filed by the Company with the SEC on March 17, 2023 with respect to the disclosure and Friedman LLP’s letter to the SEC as required under this Item 16F.
Item 16G. CORPORATE GOVERNANCE
As a Cayman Islands company listed on Nasdaq, we are subject to the Nasdaq corporate governance listing standards. However, the Nasdaq Stock Market listing rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Other than as described in this section, our corporate governance practices do not differ from those followed by domestic companies listed on the Nasdaq Capital Market. Nasdaq Stock Market listing rule 5635 generally provides that shareholder approval is required for U.S. domestic companies listed on the Nasdaq Capital Market prior to issuance (or potential issuance) of securities (i) equaling 20% or more of the company’s common stock or voting power for less than the greater of market or book value (ii) resulting in a change of control of the company; and (iii) which is being issued pursuant to a stock option or purchase plan to be established or materially amended or other equity compensation arrangement made or materially amended. Notwithstanding this general requirement, Nasdaq Stock Market listing rule 5615(a)(3)(A) permits foreign private issuers to follow their home country practice rather than these shareholder approval requirements. The Cayman Islands do not require shareholder approval prior to any of the foregoing types of issuances. Our company, therefore, is not required to obtain such shareholder approval prior to entering into a transaction with the potential to issue securities as described above. Our board of directors has elected to follow the Company’s home country rules as to such issuances and will not be required to seek shareholder approval prior to entering into such a transaction.
Item 16H. MINE SAFETY DISCLOSURE
Not applicable.
Item 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
93
ITEM 16J. INSIDER TRADING POLICIES
Regencell Bioscience Holdings’
board of directors has
ITEM 16K. CYBERSECURITY
Risk Management and Strategy
We recognize the importance of safeguarding the security of our computer systems, software, networks, and other technology assets. We have implemented cybersecurity measures and protocols for assessing, identifying, and managing material risks from cybersecurity threats, which are integrated into our overall risk management framework. We aim to ensure a comprehensive and proactive approach to safeguarding our assets and operations.
As of the date of this annual report, we have not experienced any material cybersecurity incidents or identified any material cybersecurity threats that have affected or are reasonably likely to materially affect us, our business strategy, results of operations or financial condition.
Governance
Our board of directors is responsible for overseeing risks related to cybersecurity. Our board of directors shall (i) maintain oversight of the disclosure related to cybersecurity matters in current reports or periodic reports of our company, (ii) review updates to the status of any material cybersecurity incidents or material risks from cybersecurity threats to our company, and the disclosure issues, if any, presented by our management on a quarterly basis, and (iii) review disclosure concerning cybersecurity matters in our annual report on Form 20-F presented by our management.
At the management level, our CBO and COO and information technology personnel are responsible for assessing, identifying and managing cybersecurity risks and monitoring the prevention, detection, mitigation, and remediation of cybersecurity incidents. Our CBO and COO report to our board of directors (i) timely updates to the status of any material cybersecurity incidents or material risks from cybersecurity threats to our company, and the disclosure issues, if any, and (ii) in connection with disclosure concerning cybersecurity matters in our annual report on Form 20-F.
If a cybersecurity incident occurs, our information technology personnel will promptly organize an internal assessment. If it is further determined that the incident could potentially be a material cybersecurity event, the cybersecurity-related departments will promptly report the incident and assessment results to our CBO and COO, and, to the extent appropriate, seek advice from external experts and legal counsels. If it is determined that the incident could potentially be a material cybersecurity event, our CBO and COO will decide on relevant response measures and management shall promptly prepare disclosure material on the cybersecurity incident for review and approval by our board of directors before it is disseminated to the public.
94
Part III
Item 17. FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18.
Item 18. FINANCIAL STATEMENTS
The consolidated financial statements of Regencell Bioscience Holdings Limited, and its subsidiaries are included at the end of this Annual Report.
Item 19. EXHIBITS
EXHIBIT INDEX
95
| * | Filed herewith. |
| ** | Furnished herewith. |
96
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
| Regencell Bioscience Holdings Limited | ||
| By: | /s/ Yat-Gai Au | |
| Name: | Yat-Gai Au | |
| Title: | Chief Executive Officer (Principal Executive Officer) | |
| Dated: | October 25, 2024 | |
97
REGENCELL BIOSCIENCE HOLDINGS LIMITED
Index to Consolidated Financial Statements
F-1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Regencell Bioscience Holdings Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Regencell Bioscience Holdings Limited (the “Company”) as of June 30, 2024 and 2023, the related consolidated statements of operations and comprehensive loss, changes in shareholders’ equity and cash flows for each of the years in the two-year period ended June 30, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2024 and 2023, and the results of its operations and its cash flows for each of the years in the two-year period ended June 30, 2024, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/
Marcum Asia CPAs LLP
We have served as the Company’s auditor since 2020. (such date takes into account the acquisition of certain assets of Freidman LLP by Marcum Asia CPAs LLP effective September 1, 2022)
October 25, 2024
NEW YORK OFFICE • 7 Penn Plaza • Suite
830 • New York, New York • 10001
Phone 646.442.4845 • Fax 646.349.5200 • www.marcumasia.com
F-2

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Regencell Bioscience Holdings Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated statements of operations and comprehensive loss, changes in shareholders’ equity (deficit), and cash flows of Regencell Bioscience Holdings Limited and Subsidiaries (collectively, the “Company”) for the year ended June 30, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the results of its operations and its cash flows for the year ended June 30, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/
Friedman LLP
We served as the Company’s auditor from 2020 through 2022.
October 31, 2022

F-3
REGENCELL BIOSCIENCE HOLDINGS LIMITED AND SUBSIDIARIES
CONSOLIDATED Balance sheets
| As of June 30, | ||||||||
| 2024 | 2023 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Short-term investment | ||||||||
| Prepayments and other receivables | ||||||||
| Total current assets | ||||||||
| OTHER ASSETS | ||||||||
| Property and equipment, net | ||||||||
| Long-term deposits | ||||||||
| Right-of-use assets, net | ||||||||
| Total other assets | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accrued expenses | ||||||||
| Other payables – related parties | ||||||||
| Operating lease liabilities – current | ||||||||
| Total current liabilities | ||||||||
| NON-CURRENT LIABILITIES | ||||||||
| Operating lease liabilities – non – current | ||||||||
| Total liabilities | ||||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary shares, $ | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| Total equity attributed to shareholders of the Company | ||||||||
| Non-controlling interest | ( | ) | ||||||
| Total shareholders’ equity | ||||||||
| Total liabilities and shareholders’ equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
REGENCELL BIOSCIENCE HOLDINGS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| For the Year Ended June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| OPERATING EXPENSES: | ||||||||||||
| Selling and marketing | $ | $ | $ | |||||||||
| General and administrative (including share-based compensation of $ | ||||||||||||
| Research and development (including reversal of share-based compensation of $ | ||||||||||||
| Total operating expenses | ||||||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ||||||
| OTHER INCOME, NET | ||||||||||||
| LOSS BEFORE INCOME TAXES | ( | ) | ( | ) | ( | ) | ||||||
| PROVISION FOR INCOME TAXES | ||||||||||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| NET LOSS ATTRIBUTABLE TO: | ||||||||||||
| Shareholders of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Non-controlling interest | ( | ) | ( | ) | ( | ) | ||||||
| $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| OTHER COMPREHENSIVE LOSS | ||||||||||||
| Foreign currency translation adjustment | ( | ) | ||||||||||
| COMPREHENSIVE LOSS | $ | ( | ) | ( | ) | $ | ( | ) | ||||
| NET COMPREHENSIVE LOSS ATTRIBUTABLE TO: | ||||||||||||
| Shareholders of the Company | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Non-controlling interest | ( | ) | ( | ) | ( | ) | ||||||
| $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| WEIGHTED AVERAGE NUMBER OF ORDINARY SHARES | ||||||||||||
| Basic and diluted | ||||||||||||
| LOSS PER SHARE | ||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
REGENCELL BIOSCIENCE HOLDINGS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF changeS in SHAREHOLDERS’ EQUITY (DEFICIT)
| Ordinary Shares | Additional paid-in | Accumulated Other comprehensive | Accumulated | Non- controlling | ||||||||||||||||||||||||
| Shares | Par value | Capital | loss | deficit | interest | Total | ||||||||||||||||||||||
| BALANCE, June 30, 2021 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||||||||||||
| Issuance of shares upon initial public offering and over allotment, net | ||||||||||||||||||||||||||||
| Issuance of warrants | - | |||||||||||||||||||||||||||
| Issuance of shares upon conversion of convertible bonds | ||||||||||||||||||||||||||||
| Share-based compensations | - | |||||||||||||||||||||||||||
| Capital contribution from non-controlling interest of a subsidiary | - | |||||||||||||||||||||||||||
| Exercise of warrants | ( | ) | ||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| BALANCE, June 30, 2022 | $ | $ | $ | $ | ( | ) | $ | $ | ||||||||||||||||||||
| Share-based compensations | - | |||||||||||||||||||||||||||
| Capital contribution from non-controlling interest of a subsidiary | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||||||
| BALANCE, June 30, 2023 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||
| Share-based compensations | - | |||||||||||||||||||||||||||
| Capital contribution from non-controlling interest of a subsidiary, net | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| Foreign currency translation adjustment | - | |||||||||||||||||||||||||||
| BALANCE, June 30, 2024 | $ | $ | $ | ( | ) | $ | ( | ) | $ | $ | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
REGENCELL BIOSCIENCE HOLDINGS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF cash flow
| For the Year Ended June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | ||||||||||||
| Amortization of right-of-use asset | ||||||||||||
| Share-based compensation | ||||||||||||
| Interest income | ( | ) | ( | ) | ( | ) | ||||||
| Loss on disposal of property and equipment | ||||||||||||
| Change in operating assets and liabilities | ||||||||||||
| Prepayments and other receivables | ( | ) | ( | ) | ( | ) | ||||||
| Long-term deposits | ( | ) | ||||||||||
| Accrued expenses | ( | ) | ( | ) | ||||||||
| Operating lease liabilities | ( | ) | ( | ) | ( | ) | ||||||
| Other payables – related parties | ( | ) | ( | ) | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ( | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of property and equipment | ( | ) | ( | ) | ( | ) | ||||||
| Proceed from disposal of property and equipment | ||||||||||||
| Proceed from mature of short-term investment | ||||||||||||
| Placement of short-term investment | ( | ) | ( | ) | ( | ) | ||||||
| Net cash provided by (used in) investing activities | ( | ) | ||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Repayment of proceeds from shareholder’s loans | ( | ) | ||||||||||
| Proceeds from initial public offering, net | ||||||||||||
| Capital contribution from non-controlling interest of a subsidiary, net | ||||||||||||
| Net cash provided by financing activities | ||||||||||||
| EFFECT OF EXCHANGE RATE ON CASH | ( | ) | ||||||||||
| NET CHANGE IN CASH | ( | ) | ||||||||||
| CASH, beginning of year | ||||||||||||
| CASH, end of year | $ | $ | $ | |||||||||
F-7
REGENCELL BIOSCIENCE HOLDINGS LIMITED AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF cash flow (Continued)
| SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||||||
| Cash paid for income tax | $ | $ | $ | |||||||||
| Cash paid for interest | $ | $ | $ | |||||||||
| Supplemental disclosure of non-cash financing activities: | ||||||||||||
| Conversion of convertible note payable – related party to ordinary shares | $ | $ | $ | |||||||||
| Issue of warrants | ||||||||||||
| Deferred IPO costs reclassed to Additional paid-in capital | ||||||||||||
| Right-of-use assets obtained in exchange of lease liabilities | ||||||||||||
| Early termination of right-of-use assets in exchange of lease liabilities | ( | ) | ||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Note 1 – Nature of business and organization
Regencell Bioscience Holdings Limited (“Regencell” or the “Company”) is a holding company incorporated as an exempted company on October 30, 2014, under the laws of the Cayman Islands and holding all of the outstanding share capital of Regencell Limited, established under the laws of Hong Kong on November 20, 2014, and Regencell Bioscience Limited, established under the laws of Hong Kong on May 12, 2015.
The Company, through its controlling interests in the wholly owned subsidiaries, operates an early-stage bioscience company that focuses on research, development and commercialization of Traditional Chinese Medicine (“TCM”) for the global treatment of neurocognitive disorder and degeneration, specifically for Attention Deficit Hyperactivity Disorder (“ADHD”) and Autism Spectrum Disorder (“ASD”). The Company is in the research and development stage and has not yet generated any revenue since inception.
In January 2018, the Company entered into Strategic Partnership Agreement with Mr. Sik-Kee Au (the “TCM Practitioner”), the father of Mr. Yat-Gai Au, CEO and director of the Company. Pursuant to the Strategic Partnership Agreement, the Company has exclusive rights including the intellectual property rights and ownership of all his TCM formulae. Any inventions, TCM formulae, utilities, models’ improvements, research, discoveries, designs, processes, methods of manufacture, and products conceived or made by the TCM Practitioner in relation to TCM shall be the sole and exclusive property of the Company.
The Strategic Partnership Agreement does not have
a termination date and will remain effective indefinitely under the Hong Kong law. The Strategic Partnership Agreement can be amended
or terminated by the mutual written agreement of the parties without breach. Pursuant to the Strategic Partnership Agreement, in exchange
for the rights, the Company is required to donate three percent (
On July 20, 2021, the Company consummated its
Initial Public Offering (“IPO”) of
On September 2, 2021, the Company’s wholly-owned
subsidiary, Regencell Bioscience Limited entered into a joint venture agreement with Honor Epic Enterprises Limited to form a joint venture,
Regencell Bioscience Asia Limited, under the laws of Hong Kong. The Company held
The Company has funded its operations primarily
from the net proceeds from the completion of its IPO.
| Name of Entity | Principal Activities | Place and Date of Incorporation | Ownership | |||
| Regencell Bioscience Limited | ||||||
| Regencell Limited | Production and testing laboratory Research and Development | |||||
| Regencell Bioscience Asia Limited | ||||||
| Regencell Bioscience North America Limited |
F-9
Note 2 – Summary of significant accounting policies
Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) pursuant to the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”).
Principles of consolidation
The consolidated financial statements include the financial statements of the Company and its subsidiaries. All intercompany transactions and balances among the Company and its subsidiaries have been eliminated upon consolidation.
Subsidiaries are those entities in which the Company, directly or indirectly, controls more than one half of the voting power; or has the power to govern the financial and operating policies, to appoint or remove the majority of the members of the board of directors, or to cast a majority of votes at the meeting of directors.
Non-controlling interests in the results and equity of subsidiaries are shown separately in the consolidated income statement, statement of comprehensive income, statement of changes in equity and balance sheet, respectively.
Use of estimates and assumptions
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant accounting estimates reflected in the Company’s consolidated financial statements required to be made by management include, but not limited to, the useful lives of property and equipment, impairment of long-lived assets, valuation of share options, share-based compensation, allowance for deferred tax assets and uncertain tax position. Actual results could differ from these estimates.
Risks and Uncertainties
The Company operates in an industry that is subject to intense competition, government regulations and rapid technological changes. Operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory, and other risks, including the potential risk of business failure.
Segment
Operating segments are defined as components of
an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker
(“CODM”), or decision making group, in deciding how to allocate resources and in assessing performance. The CODM is comprised
of certain members of the Company’s management team. The Company has
Foreign currency translation and transaction
The reporting currency of the Company is the United States dollar (“$”). The Company conducts its businesses in Hong Kong in the local currency, Hong Kong Dollar (“HK$”), as its functional currency. The consolidated balance sheet accounts, statement of operations accounts and equity accounts are all translated at the exchange rate as quoted by the Hong Kong Monetary Authority (“HKMA”). The Company considers the foreign exchange risk in relation to transactions denominated in HK$ with respect to $ is not significant as HK$ is pegged to $.
F-10
Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at the rates of exchange existing at the balance sheet date. Transactions in currencies other than the functional currency during the year are converted into the functional currency at the applicable rates of exchange prevailing at the transaction date. Transaction gains and losses are recognized in “other income, net”. Assets and liabilities denominated in foreign currencies are translated at the exchange rates at the balance sheet date. Equity accounts are translated at historical exchange rates and revenues, expenses, gains and losses are translated using the average rate for the year. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component of other comprehensive loss in the consolidated statements of changes in shareholders’ deficit and comprehensive loss.
Cash
Cash represents demand deposits placed with banks
or other financial institutions, which are unrestricted as to withdrawal or use, and which have original maturities of three months or
less and are readily convertible to known amounts of cash. All of the cash balances as at June 30, 2024 and 2023, were maintained at financial
institutions in Hong Kong, and were insured by the Hong Kong Deposit Protection Board up to a limit of HK $
Short term investment
Short term investment represents certificates of bank deposits or time deposits that have a maturity, at the date of purchase, of less than one year.
Property and equipment, net
Property and equipment are stated at cost less
accumulated depreciation and impairment loss, if any.
| Useful Life | ||
| Leasehold improvement | Shorter of the remaining lease terms or estimated useful lives | |
| Office furniture, equipment and others | ||
| Motor vehicles |
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts and any gain or loss is included in the consolidated statements of operations and comprehensive loss. Expenditures for maintenance and repairs are charged to earnings as incurred, while additions, renewals and betterments, which are expected to extend the useful life of assets, are capitalized. The Company also re-evaluates the periods of depreciation to determine whether subsequent events and circumstances warrant revised estimates of useful lives.
Prepayment
Prepayments primarily consist of prepayments of corporate apartments’ rental. Prepayments are classified as current based on the terms of the respective agreements. This prepayment is unsecured and is reviewed periodically to determine whether their carrying value has become impaired.
Long-term deposits
Long-term deposits present the deposits paid to the landlords as required on the signing of the various lease agreements and thereafter, to be held by the landlords as security for the performance of the Company’s obligations under the lease agreements. The deposits are classified as either current or non-current based on the terms of the respective agreements. The deposit is unsecured and is reviewed periodically to determine whether its carrying value has become impaired.
F-11
Impairment for long-lived assets
Long-lived assets, including property and equipment with finite lives are reviewed for impairment whenever events or changes in circumstances (such as a significant adverse change to market conditions that will impact the future use of the assets) indicate that the carrying value of an asset may not be recoverable. The Company assesses the recoverability of the assets based on the undiscounted future cash flows the assets are expected to generate and recognize an impairment loss when estimated undiscounted future cash flows expected to result from the use of the asset plus net proceeds expected from disposition of the asset, if any, are less than the carrying value of the asset. If an impairment is identified, the Company would reduce the carrying amount of the asset to its estimated fair value based on a discounted cash flows approach or, when available and appropriate, to comparable market values. For the years ended June 30, 2024, 2023 and 2022, no impairment loss on long-lived assets was recognized.
Fair value measurement
ASC 825-10 requires certain disclosures regarding the fair value of financial instruments. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy prioritizes the inputs used to measure fair value. The hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| ● | Level 1 — inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 — inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, quoted market prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable and inputs derived from or corroborated by observable market data. |
| ● | Level 3 — inputs to the valuation methodology are unobservable. |
Unless otherwise disclosed, the fair value of the Company’s financial instruments including cash, short-term investment, accrued expenses and other payables – related parties approximates their recorded values due to their short-term maturities. The fair value of the long-term deposits approximates their carrying amounts because the deposit was paid in cash.
Selling and Marketing Expenses
Sales and marketing expenses were mainly for marketing
initiatives and sponsorship with Non-Governmental Organizations (“NGOs”) and institutions that serves families with ADHD and
ASD children, who voluntary sign up as qualified patients to be enrolled in our efficacy trial in the future. The Group expenses all advertising
costs as incurred and classifies these costs under sales and marketing expenses. For the years ended June 30, 2024, 2023 and 2022, the
advertising expenses were approximately $
Research and development Expenses
Research and development costs are charged to operations as incurred. The Company accounts for nonrefundable advance payments for goods and services that will be used in future research and development activities as expenses when the goods have been received or when the service has been performed rather than when the payment is made. Research and development expenses consist of costs incurred by the Company for the discovery and development of the Company’s product candidates. Research and development costs include, but not limited to, payroll and personnel expenses including share-based compensation, research studies supplies, fees for research studies services, consulting costs, and allocated overhead, including rent, equipment, and utilities.
F-12
Employee benefit plan
Employees of the Company located in Hong Kong
participate in a compulsory saving scheme (pension fund) for the retirement of residents in Hong Kong. Employees are required to contribute
monthly to mandatory provident fund schemes provided by approved private organizations, according to their salaries and the period of
employment. The Company is required to contribute to the plan based on certain percentages of the employees’ salaries, up to a maximum
amount specified by the local government. For the years ended June 30, 2024, 2023 and 2022, the contribution made was approximately $
Leases
The Company adopted the new lease accounting standard – ASC 842 on July 1, 2021. The Company determines if an arrangement is or contains a lease at inception. Operating leases with lease terms of more than 12 months are included in operating lease assets, accrued and other current liabilities, and long-term operating lease liabilities on its consolidated balance sheet. Operating lease assets represent its right to use an underlying asset for the lease term and lease liabilities represent its obligation to make lease payments over the lease term. Operating lease assets and liabilities are recognized based on the present value of the remaining lease payments discounted using its incremental borrowing rate. Lease expense is recognized on a straight-line basis over the lease term.
Related Parties
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operational decisions. Companies are also considered to be related if they are subject to common control or common significant influence, such as a family member or relative, shareholder, or a related corporation.
Government grants
Government grants include financial incentives in the form of cash subsidies that involve no conditions or continuing performance obligations of the Company. Government grants are recognized as other non-operating income upon receipt.
Share-based compensation
The Company accounts for share-based compensation in accordance with ASC 718, Compensation — Stock Compensation (“ASC 718”). The Company determines whether an award should be classified and accounted for as a liability award or an equity award. All of the Company’s share-based awards were classified as equity awards and are recognized in the consolidated financial statements based on their grant date fair values.
The Company has elected to recognize share-based
compensation using the straight-line method for all share-based awards granted with graded over the requisite service period, which is
the vesting period. The Company accounts for forfeitures as they occur in accordance with ASU No. 2016-09, Compensation — Stock
Compensation (Topic 718): Improvement to Employee Share-based Payment Accounting. The Company, with the assistance of an independent third-party
valuation firm, determined the fair value of the share options granted to employees. The Black Scholes Model and/or the Binomial Model
was applied in determining the estimated fair value of the options granted to employees and non-employees. During the years ended June
30, 2024, 2023 and 2022,
F-13
Income taxes
The Company accounts for current income taxes in accordance with the laws of the relevant tax authorities. Deferred income taxes are recognized when temporary differences exist between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
An uncertain tax position is recognized as a benefit
only if it is “more likely than not” that the tax position would be sustained in a tax examination. The amount recognized
is the largest amount of tax benefit that is greater than
(Loss) earnings per share
The Company computes (loss) earnings per share (“EPS”) in accordance with ASC 260, “Earnings per Share”. ASC 260 requires companies to present basic and diluted EPS. Basic EPS is measured as net income (loss) divided by the weighted average ordinary share outstanding for the period. Diluted income per share is calculated by dividing net income attributable to the holders of ordinary shares as adjusted for the effect of dilutive ordinary share equivalents, if any, by the weighted average number of ordinary shares and dilutive ordinary share equivalents outstanding during the period. Potential Ordinary Shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. For the years ended June 30, 2024, 2023 and 2022, there were no dilutive shares.
Commitments and Contingencies
In the normal course of business, the Company is subject to contingencies, including legal proceedings and claims arising out of the business that relate to a wide range of matters, such as government investigations and tax matters. The Company recognizes a liability for such contingency if it determines it is probable that a loss has occurred and a reasonable estimate of the loss can be made. The Company may consider many factors in making these assessments including historical and the specific facts and circumstances of each matter.
Concentration of credit risk
Financial instruments that potentially subject the Company to concentration of credit risks consist primarily of cash, the balances of which are stated on the consolidated balance sheets which represent the Company’s maximum exposure.
The Company places its cash in good credit quality financial institutions in Hong Kong. The Company deposits its cash in financial institutions that it believes have high credit quality and have not experienced any losses on such accounts and does not believe it is exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships.
Recently issued accounting pronouncements
In December 2023, the FASB issued ASU 2023-09 “Improvements to Income Tax Disclosures” (“ASU 2023-09”). ASU 2023-09 intends to improve the transparency of income tax disclosures. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024 and is to be adopted on a prospective basis with the option to apply retrospectively. The Company is currently evaluating the impact from the adoption of this ASU on its consolidated financial statements.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires business entities to enhance disclosures about significant segment expenses. The ASU also requires that a public entity with a single reportable segment, like the Company, provide all of the disclosures required as part of the amendment and all existing disclosures required by Topic 280. The ASU will become effective for us for the annual period beginning in fiscal year 2025 and for interim periods beginning with our 2026 fiscal year, on a retrospective basis, and early adoption is permitted. The Company is currently evaluating the effect that the new guidance will have on our related disclosures, but do not expect this guidance will have a material impact on the Company’s financial position and results of operations.
F-14
Except as mentioned above, the Company does not believe other recently issued but not yet effective accounting standards, if currently adopted, would have a material effect on the Company’s consolidated balance sheets, statements of operations and comprehensive income and statements of cash flows.
Note 3 – Property and equipment, net
| June 30, 2024 | June 30, 2023 | |||||||
| Leasehold improvement | $ | $ | ||||||
| Office furniture, equipment and others | ||||||||
| Motor vehicles | ||||||||
| Total | ||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Depreciation expense for the years ended June
30, 2024, 2023 and 2022, amounted to approximately $
During the years ended June 30, 2024, 2023 and
2022, there was a loss on disposal of property and equipment amounted to approximately , $
Note 4 – Accrued expenses
Accrued expenses represent salary and welfare payables, professional fees, utilities, and payables for other operating expenses.
Note 5 – Related Party Transaction
Other payables – related parties
During the year ended June 30, 2022, the Company
has an office rental agreement with Ace United International Limited, which is a company wholly owned by the Company’s Founder and
CEO. The Company has paid a monthly rent of $
During the years ended June 30, 2024 and 2023,
other payables – related parties represent reimbursement from related parties and the Company’s executive management, Mr.
Chung and Mr. Au for out-of-pocket expenses incurred for business purposes. The balances due to Mr. Chung and Mr. Au was approximately
$
During the years ended June 30, 2024 and 2023,
the expenses paid for TCM formulae and materials for product development to Regeneration Company Limited, a related company, were approximately
$
F-15
During the years ended June 30, 2024 and 2023,
the expenses paid for services fee for research support to Regencell Bioscience Asia Sdn. Bhd., a related company, were approximately
$
Note 6 – Taxes
Income tax
| For the year ended June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Current: | ||||||||||||
| Hong Kong | $ | $ | $ | |||||||||
| Deferred: | ||||||||||||
| Hong Kong | ||||||||||||
| Total provision for income taxes | ||||||||||||
Cayman Islands and British Virgin Islands (“BVI”)
Under the current laws of the Cayman Islands and BVI, the Company and its subsidiaries are not subject to tax on income or capital gains. Additionally, upon payments of dividends to the shareholders, no Cayman Islands or BVI withholding tax will be imposed.
Hong Kong
Entities incorporated in Hong Kong are subject
to Hong Kong profits tax at a rate of
| For the year ended June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Tax benefit calculated at statutory tax rate | % | % | % | |||||||||
| Valuation allowance | ( | )% | ( | )% | ( | )% | ||||||
| Effective tax rate | % | % | % | |||||||||
| As of June 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Deferred tax assets: | ||||||||||||
| Net operating loss carry forwards | $ | $ | $ | |||||||||
| Share-based compensation expenses | ||||||||||||
| Less: valuation allowance | ( | ) | ( | ) | ( | ) | ||||||
| Deferred tax asset, net | $ | $ | $ | |||||||||
F-16
| For the year ended June 30, | ||||||||
| 2024 | 2023 | |||||||
| Balance at beginning of the year | $ | $ | ||||||
| Addition | ||||||||
| Less: Reversal | ( | ) | ||||||
| Balance at end of the year | $ | $ | ||||||
The ultimate realization of deferred tax assets
is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management
considers the cumulative earnings and projected future taxable income in making this assessment. Recovery of substantially all of the
Company’s deferred tax assets is dependent upon the generation of future income, exclusive of reversing taxable temporary differences.
Based upon historical operating results and projections for future taxable income, management provided full valuation allowance for the
deferred tax assets for the years ended June 30, 2024 and 2023. As of June 30, 2024 and 2023, tax loss carry-forward amounted to approximately
$
The Company evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measures the unrecognized benefits associated with the tax positions. As of June 30, 2024, and 2023, the Company did not have any significant unrecognized uncertain tax positions. The Company did not incur any interest and penalties related to potential underpaid income taxes for the years ended June 30, 2024 and 2023. The Company’s major tax jurisdiction is Hong Kong. The tax years 2018 through 2024 remain subject to examination by the Hong Kong Inland Revenue Department (the “HKIRD”). The Company also does not anticipate any significant increases or decreases in unrecognized tax benefits in the next 12 months from June 30, 2024.
Note 7 – Shareholder’s equity (deficit)
Ordinary Shares
Regencell Bioscience Holdings Limited (Cayman)
was established under the laws of Cayman Islands on October 30, 2014. The authorized number of Ordinary Shares is
Initial Public Offering
On July 20, 2021, the Company consummated its
IPO of
2021 Share Option Plan
On May 31, 2021, the Company adopted a 2021 Share Option Plan (the “Plan”). The Plan is a share-based compensation plan that provides for discretionary grants of share options to key employees, directors and consultants of the Company. The purpose of the Plan is to recognize contributions made to the Company and its subsidiaries by such individuals and to provide them with additional incentive to achieve the objectives of the Company.
F-17
On June 9, 2021, the board of the Company granted
issuance of
On January 1, 2022, the board of the Company granted
issuance of
The Plan became effective on the date of the Company’s initial public offering, which was July 20, 2021. It shall continue in effect for a term of ten (10) years unless sooner terminated or unless renewed for another period not to exceed ten (10) years pursuant to shareholder approval. It is intended that share options qualify as “performance-based compensation”.
Share-based Compensation
The Company has elected to recognize share-based compensation expense using a straight-line method for the entire employee equity awards granted with graded vesting based on service conditions provided that the amount of compensation cost recognized at any date is at least equal to the portion of the grant-date value of the equity awards that are vested at that date.
The fair value of share options was determined at the date of grant using the Black-Scholes option pricing model or the Binomial option pricing model.
The Black-Scholes option pricing model requires management to make various estimates and assumptions, including expected term, expected volatility, risk-free rate, and dividend yield. The Binomial Option Pricing model requires management to make various estimates and assumptions, including the grant date share price, expected volatility, expected early exercise multiple, option life, risk-free interest rate and dividend yield.
F-18
For the years ended June 30, 2024 and 2023, key inputs used to estimate the fair value of share options on the grant dates are as follows:
| Options granted in June 2021 | ||||
| Risk-free interest rate | % | |||
| Expected life of the options | ||||
| Expected volatility | % | |||
| Expected dividend yield | % | |||
| Fair value | $ | |||
| Options granted in January 2022 |
||||
| Fair value of the ordinary shares on the date of option grant | $ | |||
| Risk-free interest rate | % | |||
| Option life | ||||
| Expected volatility | % | |||
| Expected dividend yield | % | |||
| Exercise price | $ |
|||
| Expected early exercise multiple | ||||
| Number of Share Options | Weighted Average Exercise Price | Weighted Average Grant-date Fair value | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | ||||||||||||||||
| $ | $ | Year | $ | |||||||||||||||||
| Outstanding as of July 1, 2022 | ||||||||||||||||||||
| Granted | ||||||||||||||||||||
| Expired, forfeited or cancelled | ( | ) | ||||||||||||||||||
| Outstanding as of June 30, 2023 | ||||||||||||||||||||
| Granted | ||||||||||||||||||||
| Expired, forfeited or cancelled | ( | ) | ||||||||||||||||||
| Outstanding as of June 30, 2024 | ( | ) | ||||||||||||||||||
| Exercisable as of June 30, 2024 | ||||||||||||||||||||
During the year ended June 30, 2024, the Company
recognized share-based compensation expense of approximately $
F-19
Public Offering Warrants
The aggregated fair value of the Public Offering
Warrants on July 20, 2021 was $
The aggregated fair value of the overallotment
Warrants on August 19, 2021 was $
Note 8 – Leases
On July 1, 2021, we adopted ASC Topic 842 (the “new lease standard”) by applying the modified retrospective approach to all leases on July 1, 2021. Under this guidance, lessees are required to recognize assets and liabilities on the balance sheet for the rights and obligations created by all leases. We elected the package of practical expedients upon transition that permits us to not reassess (1) whether any contracts entered into prior to adoption are or contain leases, (2) the lease classification of existing leases and (3) initial direct costs for any leases that existed prior to adoption.
Upon adoption on July 1, 2021, we recognized operating
lease right-of-use (“ROU”) assets and corresponding lease liabilities of $
We determine if an arrangement is or contains a lease at inception. Our assessment is based on (1) whether the contract involves the use of a distinct identified asset, (2) whether we obtain the right to substantially all the economic benefit from the use of the asset throughout the period and (3) whether we have the right to direct the use of the asset. Leases are classified as either finance leases or operating leases. A lease is classified as a finance lease if any one of the following criteria are met: the lease transfers ownership of the asset by the end of the lease term, the lease contains an option to purchase the asset that is reasonably certain to be exercised, the lease term is for a major part of the remaining useful life of the asset or the present value of the lease payments equals or exceeds substantially all of the fair value of the asset. A lease is classified as an operating lease if it does not meet any one of these criteria. The lease classification affects the expense recognition in the statement of income. Operating lease costs are recorded entirely in operating expenses. Finance lease costs are split, where amortization of the ROU asset is recorded in operating expenses and an implied interest component is recorded in interest expense.
Under the new lease standard, operating leases (as lessee) are included in Operating lease right-of-use assets. Operating lease amortization of right of use assets, Operating lease right of use assets, net, Current operating lease liabilities and Noncurrent operating lease liabilities in our accompanying balance sheets. ROU assets and lease liabilities are recognized at commencement date based on the present value of the future minimum lease payments over the lease term. The ROU asset includes any lease payments made but excludes lease incentives and initial direct costs incurred, if any. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term.
F-20
Lease extensions
Many of our leases have options to either extend or terminate the lease. In determining the lease term, we considered all available contract extensions that are reasonably certain of occurring.
Significant assumptions and judgments
Incremental borrowing rate. As most of our leases do not provide an implicit interest rate, we use our incremental borrowing rate (“IBR”) at the commencement of the lease and estimate the IBR for each lease agreement taking into consideration lease contract term, collateral and entity credit ratings, and use sensitivity analyses to evaluate the reasonableness of the rates determined.
Lease balances and costs
All of the lease agreements that we have entered into are classified as operating leases.
Effective July 15, 2019, we entered into an agreement
to lease usage of our Hong Kong office with a third party entity. The lease was for
Effective August 2, 2021, we entered into an agreement
to lease usage of our Hong Kong office with a third party entity. The lease was for
During the years ended June 30, 2023 and 2024, we entered into agreements to lease usage of staff quarters with third parties. Each of the leases was for a two years term.
All the above leases were classified as operating
at the inception of the lease. Operating leases result in the recognition of ROU assets and lease liabilities on the balance sheet. ROU
assets and operating lease liabilities are recognized based on the present value of lease payments over the lease terms of the commencement
date. Because the leases do not provide an explicit or implicit rate of return, the Company determines incremental borrowing rate based
on the information available at the commencement date in determining the present value of lease payments on an individual lease basis.
The incremental borrowing rate for a lease is the rate of interest the Company would have to pay on a collateralized basis to borrow an
amount equal to the lease payments for the asset under similar term, which is
| Classification | Year Ended June 30, 2024 | Year Ended June 30, 2023 | ||||||||
| Operating lease costs | General and administrative expenses | $ | $ | |||||||
| Operating lease costs | Research and development expenses | |||||||||
| Short term lease costs | General and administrative expenses | |||||||||
F-21
| Classification | June 30, 2024 | June 30, 2023 | ||||||||
| Assets | ||||||||||
| Operating lease – ROU assets | Right-of-use assets | $ | $ | |||||||
| Liabilities | ||||||||||
| Operating lease liabilities | Current portion | $ | $ | |||||||
| Operating lease liabilities | Non-current portion | |||||||||
| Total lease liabilities | $ | $ | ||||||||
| Weighted average remaining term (years) | ||||||||||
| Operating leases | ||||||||||
| Weighted average discount rate | ||||||||||
| Operating leases | % | % | ||||||||
| June 30, 2024 | ||||
| For the years ending June 30, | ||||
| 2025 | $ | |||
| 2026 | ||||
| 2027 | ||||
| Total minimum lease payments | $ | |||
| Less: imputed interest | ( | ) | ||
| Total: | $ | |||
Note 9 – Commitments and contingencies
Contingencies
From time to time, the Company may be subject to certain legal proceedings, claims and disputes that arise in the ordinary course of business. Although the outcomes of these legal proceedings cannot be predicted, the Company does not believe these actions, in the aggregate, will have a material adverse impact on its financial position, results of operations or liquidity.
Note 10 – Subsequent events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the Company issued these consolidated financial statements on October 25, 2024. Based on the review, the Company did not identify any subsequent event that would have required adjustment or disclosure in the consolidated financial statements.
F-22