
Day One生物制药公司专为各年龄段人群提供靶向治疗,预计在2024年10月推出

免责声明:本演示文稿及随附的口头评论包含基于我们管理层的信念和假设以及目前可供我们管理层使用的信息的前瞻性声明。前瞻性声明本质上受到风险和不确定性的影响,其中一些无法预测或量化。在某些情况下,您可以通过“可能”、“将”、“应该”、“可以”、“期望”、“计划”、 “预计”、“相信”、“估计”、“预测”、“打算”、“潜在”、“将”、“继续”、“正在进行”或这些术语的否定形式或其他类似的术语来识别前瞻性声明。前瞻性声明包括本演示文稿中包含的除历史事实陈述之外的所有陈述,包括有关我们未来财务表现的信息, 包括我们的现金、现金及现金等价物足以资助运营的充足性,业务计划和目标,定向增发提供的预期募集总收益,商业化和市场营销努力的时间和成功,计划的非临床和临床开发活动的时间和成功,我们战略合作的成果,包括潜在的里程碑实现和在此之下提供的版税支付,非临床研究和临床试验的时间和结果,我们产品及产品候选物的疗效和安全性,OJEMDA™(托伐非尼)治疗小儿低级别神经胶质瘤(pLGG)或相关适应证的能力,我们产品及产品候选物的潜在治疗效益和经济价值,潜在增长机会,竞争地位,行业环境和潜在市场机会,我们保护知识产权的能力以及全球业务或宏观经济条件的影响,包括通货膨胀、利率变动、网络安全概念事件、全球银行系统潜在不稳定、美国总统任期更替、联邦债务上限和预算的不确定性及由此引发的潜在政府停摆和全球区域冲突对我们业务和运营的影响。前瞻性声明受到已知和未知的风险、不确定性、假设和其他因素的影响。我们的管理层无法预测所有风险,也无法评估所有因素对我们业务的影响,或者任何因素或因素的组合可能导致实际结果与我们可能提出的任何前瞻性声明中包含的结果有实质不同。这些因素以及我们最近向证券交易委员会(SEC)提交的最新10-Q表中所描述的标题“风险因素”下所描述的因素以及我们不时向SEC提交的其他文件,可能引起我们的实际结果、业绩或成就出现重大差异和不利影响,与我们前瞻性声明中预期的或暗示的结果不符。此外,“我们相信”等陈述反映了我们对相关主题的信念和看法。这些声明基于我们在此演示文稿日期可获得的信息,尽管我们认为这些信息构成这些声明的合理基础,但这些信息可能是有限或不完整的,而我们的声明不应被解释为表明我们已对所有可能可用的相关信息进行了彻底的调查或审查。这些声明本质上是不确定的,投资者被警告不要过度依赖这些声明。此外,如果我们的前瞻性声明被证明不准确,这种不准确性可能是重大的。考虑到这些前瞻性声明中的重大不确定性,您不应将这些声明视为我们或任何其他人将实现我们的目标和计划的陈述或保证,无论在任何指定的时间框架内还是根本。我们没有义务公开更新任何前瞻性声明,除非根据法律要求。本演示文稿还包含由独立方和我们制定的关于市场规模和增长以及我们所在行业的其他数据的估计和其他统计数据。这些数据涉及一系列假设和限制,我们提醒您不要过分重视这些估计。此外,对我们未来业绩和我们所在行业市场未来业绩的预测、假设和估计必然受到高度不确定性和风险的影响。

为所有年龄段的癌症患者开发药物,通过快速注册途径确立首个级别位置,在扩大到青少年和成人人群的同时,以与我们对儿童一样的承诺追求这些机会。我们的方法 纳斯达克:DAWN IPO: 2021 成立于: 2018
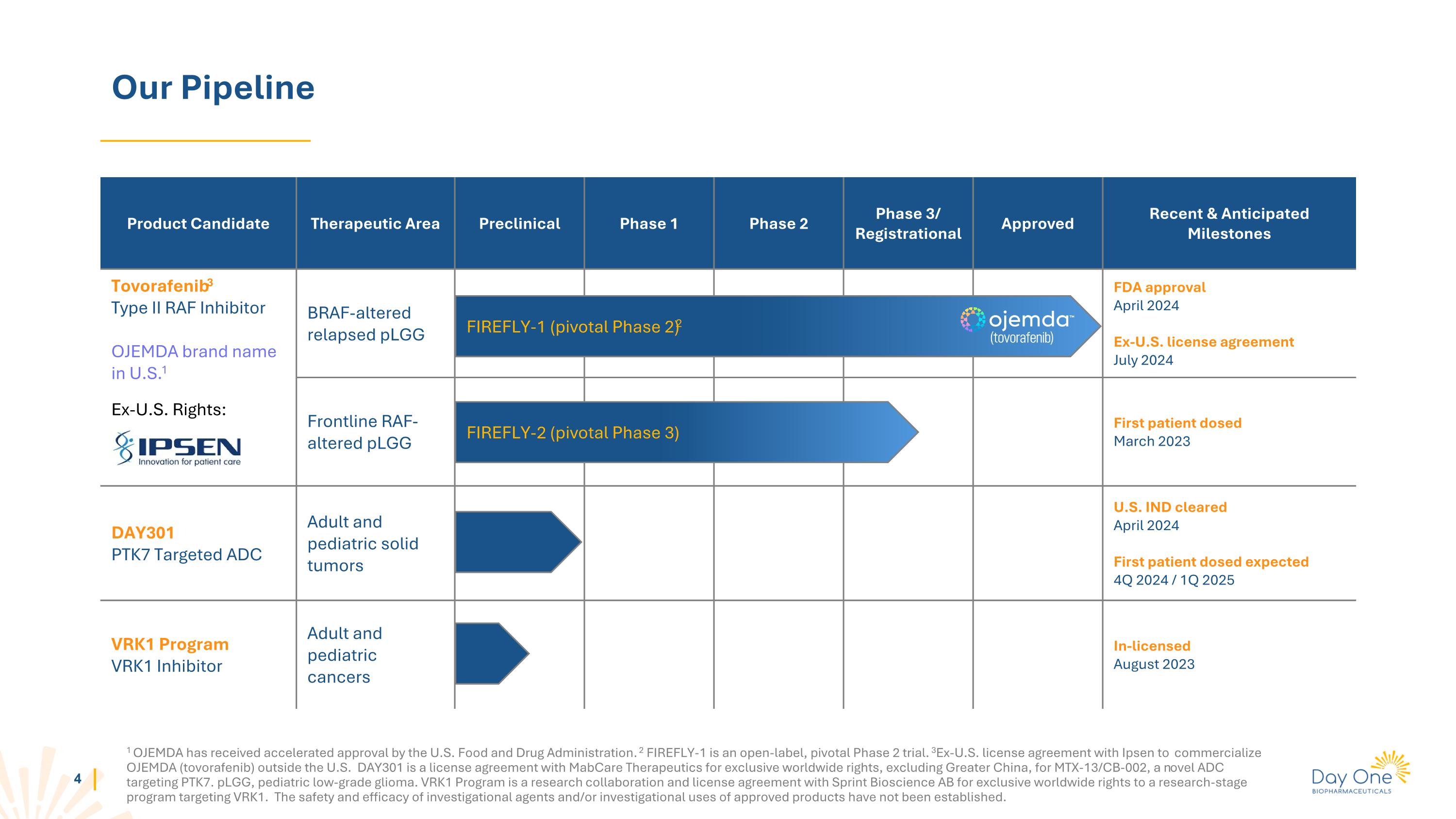
美国食品药品监督管理局已对OJEMDA进行了加速批准。FIREFLY-1是一个开放标签的关键2期试验。与爱必生签署了在美国境外商业化OJEMDA(托伐那非)的执照协议。DAY301与MabCare Therapeutics签署了独家全球权利许可协议,除了大中华地区,用于针对PTK7的新型靶向ADC MTX-13/Cb-002。pLGG,儿童低级别胶质瘤。VRK1项目是与Sprint Bioscience Ab合作的研究与许可协议,获得了针对VRK1的研究阶段项目的独家全球权利。尚未确立待研究药物和/或批准产品的安全性和有效性。我们的管线产品候选治疗领域临床前1期2期3期/注册批准最近和预期的里程碑托伐那非3型II RAF抑制剂在美国的OJEMDA品牌名称1在美国以外的权利:BRAF变异复发pLGG的FDA批准 2024年4月在美国境外的-执照协议 2024年7月的一线BRAF变异复发pLGG首名患者纳入研究 2023年3月DAY301~PTK7靶向ADC成人和儿童实体肿瘤美国IND获准 2024年4月首名患者预计纳入研究 2024年第四季度/2025年第一季度VRK1项目~VRK1抑制剂成人和儿童癌症授权 2023年8月FIREFLY-1(关键2期) FIREFLY-2(关键3期)

OJEMDATm(托伐替尼)复发或难治性BRAF改变的pLGG
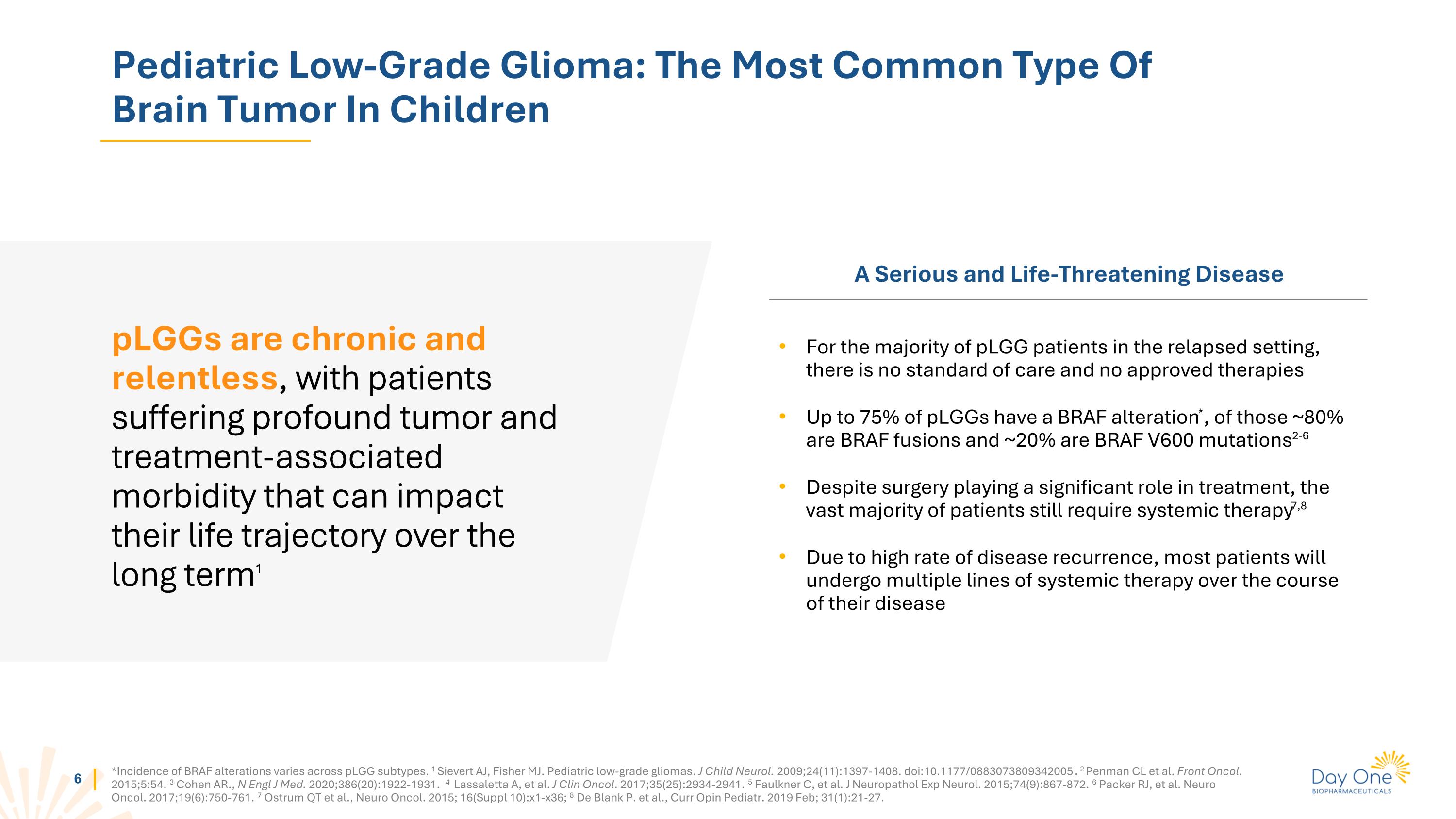
小儿低级别胶质瘤:儿童脑肿瘤中最常见的类型pLGGs是慢性和持续的,患者遭受深刻的肿瘤和与治疗相关的发病率,这可能会长期影响他们的生活轨迹1 严重且危及生命的疾病对于大多数复发的pLGG患者而言,目前没有标准治疗方法,也没有经批准的疗法高达75%的pLGG患者存在BRAF基因的改变*,其中大约80%是BRAF融合基因,约20%是BRAF V600突变2-6 尽管手术在治疗中起着重要作用,但绝大多数患者仍需要系统治疗7,8 由于疾病复发率高,大多数患者在疾病过程中将接受多线系统治疗*BRAF基因的改变发生率在pLGG亚型中各不相同。1 Sievert AJ, Fisher MJ. 小儿低级别胶质瘤。J Child Neurol. 2009;24(11):1397-1408. doi:10.1177/0883073809342005. 2 Penman CL等人。前列肿瘤。2015;5:54。 3 cohen等人,N Engl J Med。 2020;386(20):1922-1931。4 Lassaletta A等,J Clin Oncol。 2017;35(25):2934-2941。5 Faulkner C等,J Neuropathol Exp Neurol。 2015;74(9):867-872。6 Packer RJ等人。神经肿瘤。 2017;19(6):750-761。7 Ostrum Qt 等人,Neuro Oncol。 2015; 16(Suppl 10):x1-x36;8 De Blank P. 等人,Curr Opin Pediatr。 2019年2月; 31(1):21-27。
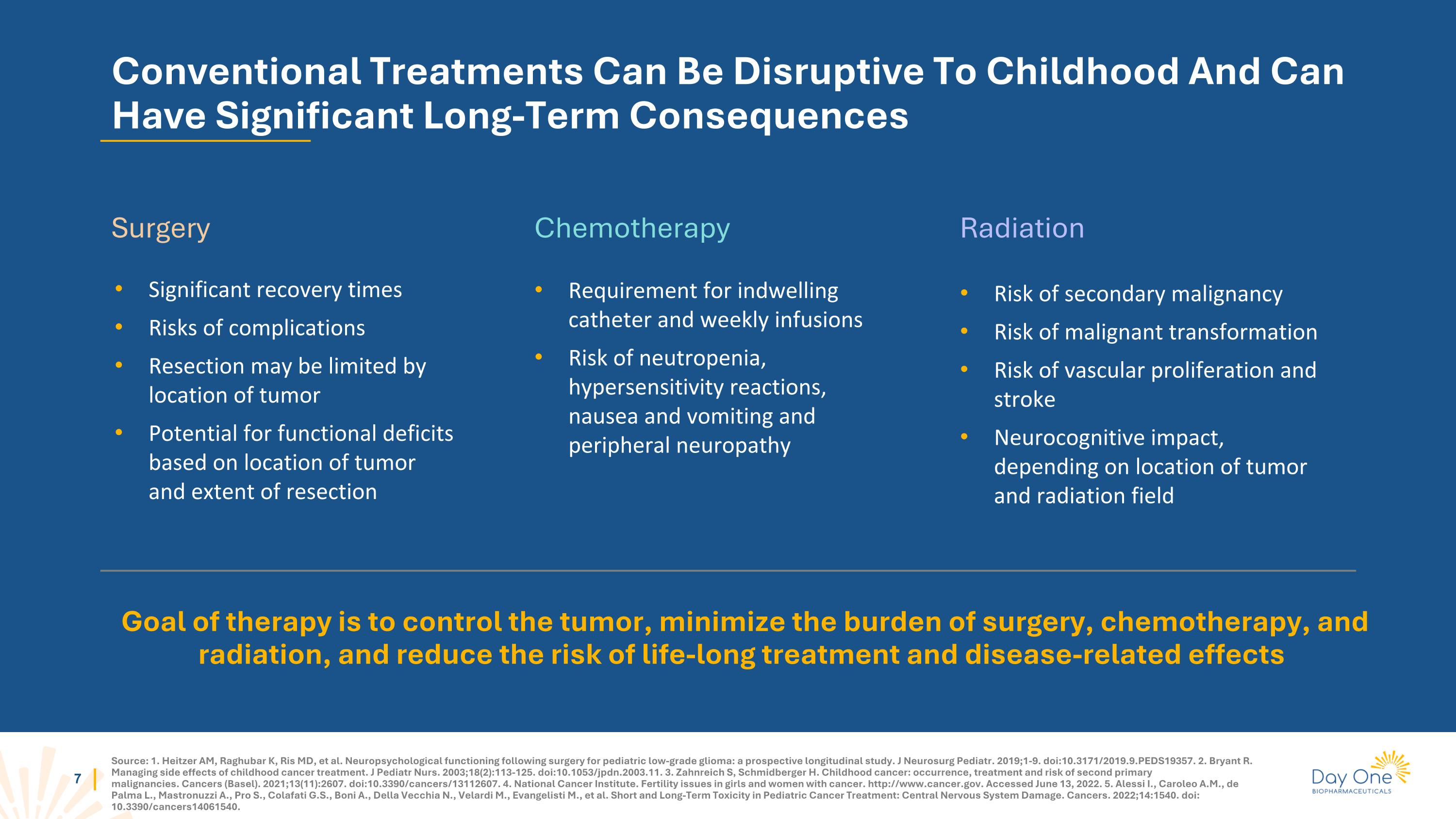
Source: 1. Heitzer AM, Raghubar K, Ris MD, et al. Neuropsychological functioning following surgery for pediatric low-grade glioma: a prospective longitudinal study. J Neurosurg Pediatr. 2019;1-9. doi:10.3171/2019.9.PEDS19357. 2. Bryant R. Managing side effects of childhood cancer treatment. J Pediatr Nurs. 2003;18(2):113-125. doi:10.1053/jpdn.2003.11. 3. Zahnreich S, Schmidberger H. Childhood cancer: occurrence, treatment and risk of second primary malignancies. Cancers (Basel). 2021;13(11):2607. doi:10.3390/cancers/13112607. 4. National Cancer Institute. Fertility issues in girls and women with cancer. http://www.cancer.gov. Accessed June 13, 2022. 5. Alessi I., Caroleo A.M., de Palma L., Mastronuzzi A., Pro S., Colafati G.S., Boni A., Della Vecchia N., Velardi M., Evangelisti M., et al. Short and Long-Term Toxicity in Pediatric Cancer Treatment: Central Nervous System Damage. Cancers. 2022;14:1540. doi: 10.3390/cancers14061540. Conventional Treatments Can Be Disruptive To Childhood And Can Have Significant Long-Term Consequences Goal of therapy is to control the tumor, minimize the burden of surgery, chemotherapy, and radiation, and reduce the risk of life-long treatment and disease-related effects Significant recovery times Risks of complications Resection may be limited by location of tumor Potential for functional deficits based on location of tumor and extent of resection Risk of secondary malignancy Risk of malignant transformation Risk of vascular proliferation and stroke Neurocognitive impact, depending on location of tumor and radiation field Requirement for indwelling catheter and weekly infusions Risk of neutropenia, hypersensitivity reactions, nausea and vomiting and peripheral neuropathy Surgery Chemotherapy Radiation
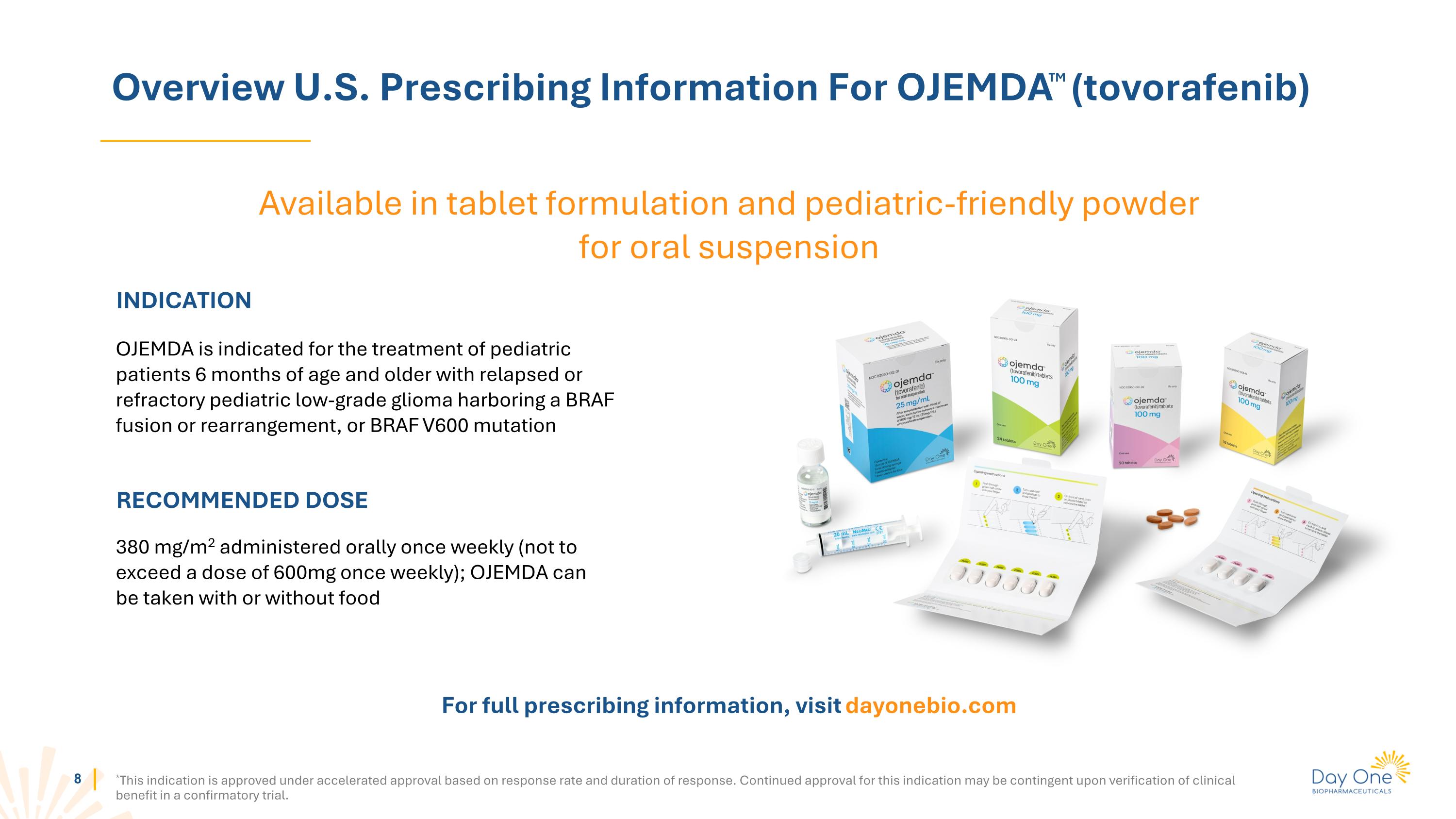
Overview U.S. Prescribing Information For OJEMDATM (tovorafenib) *This indication is approved under accelerated approval based on response rate and duration of response. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial. INDICATION OJEMDA is indicated for the treatment of pediatric patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma harboring a BRAF fusion or rearrangement, or BRAF V600 mutation RECOMMENDED DOSE 380 mg/m2 administered orally once weekly (not to exceed a dose of 600mg once weekly); OJEMDA can be taken with or without food For full prescribing information, visit dayonebio.com Available in tablet formulation and pediatric-friendly powder for oral suspension
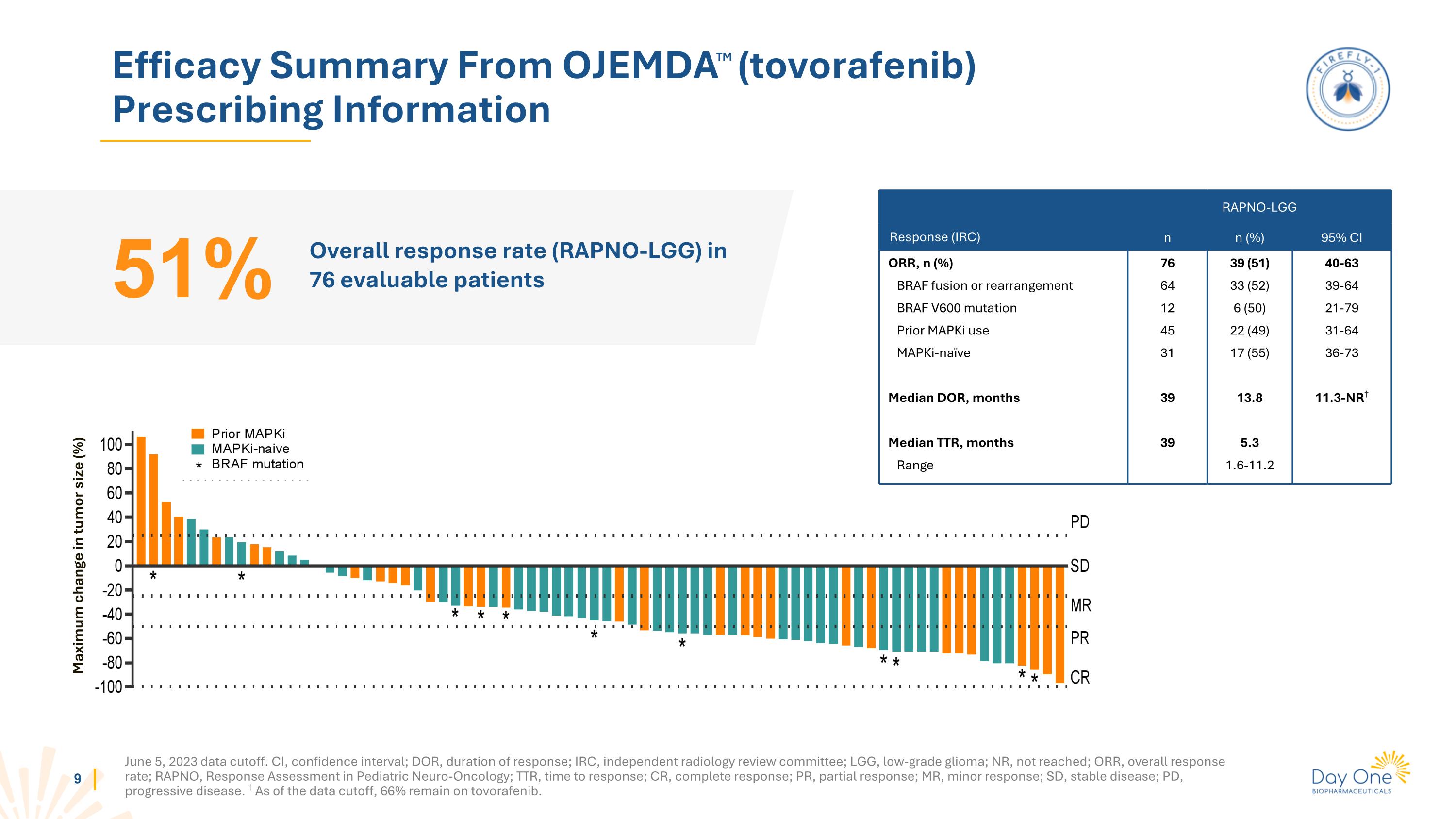
Efficacy Summary From OJEMDATM (tovorafenib) Prescribing Information Overall response rate (RAPNO-LGG) in 76 evaluable patients 51% Response (IRC) RAPNO-LGG n n (%) 95% CI ORR, n (%) BRAF fusion or rearrangement BRAF V600 mutation Prior MAPKi use MAPKi-naïve Median DOR, months Median TTR, months Range 76 64 12 45 31 39 39 39 (51) 33 (52) 6 (50) 22 (49) 17 (55) 13.8 5.3 1.6-11.2 40-63 39-64 21-79 31-64 36-73 11.3-NR† Maximum change in tumor size (%) June 5, 2023 data cutoff. CI, confidence interval; DOR, duration of response; IRC, independent radiology review committee; LGG, low-grade glioma; NR, not reached; ORR, overall response rate; RAPNO, Response Assessment in Pediatric Neuro-Oncology; TTR, time to response; CR, complete response; PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease. † As of the data cutoff, 66% remain on tovorafenib.
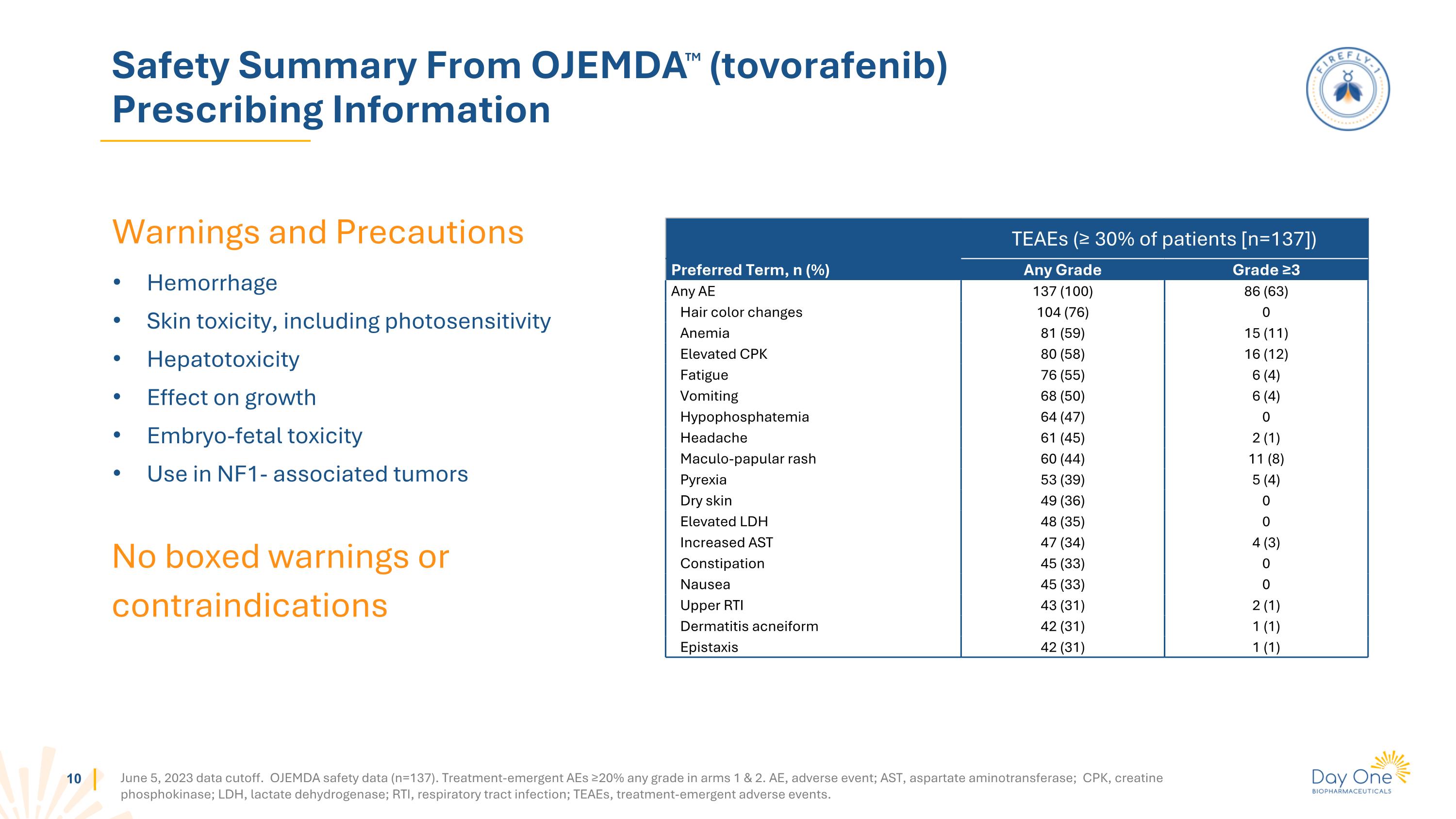
Warnings and Precautions Hemorrhage Skin toxicity, including photosensitivity Hepatotoxicity Effect on growth Embryo-fetal toxicity Use in NF1- associated tumors Safety Summary From OJEMDATM (tovorafenib) Prescribing Information No boxed warnings or contraindications TEAEs (≥ 30% of patients [n=137]) Preferred Term, n (%) Any Grade Grade ≥3 Any AE 137 (100) 86 (63) Hair color changes 104 (76) 0 Anemia 81 (59) 15 (11) Elevated CPK 80 (58) 16 (12) Fatigue 76 (55) 6 (4) Vomiting 68 (50) 6 (4) Hypophosphatemia 64 (47) 0 Headache 61 (45) 2 (1) Maculo-papular rash 60 (44) 11 (8) Pyrexia 53 (39) 5 (4) Dry skin 49 (36) 0 Elevated LDH 48 (35) 0 Increased AST 47 (34) 4 (3) Constipation 45 (33) 0 Nausea 45 (33) 0 Upper RTI 43 (31) 2 (1) Dermatitis acneiform 42 (31) 1 (1) Epistaxis 42 (31) 1 (1) June 5, 2023 data cutoff. OJEMDA safety data (n=137). Treatment-emergent AEs ≥20% any grade in arms 1 & 2. AE, adverse event; AST, aspartate aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; RTI, respiratory tract infection; TEAEs, treatment-emergent adverse events.
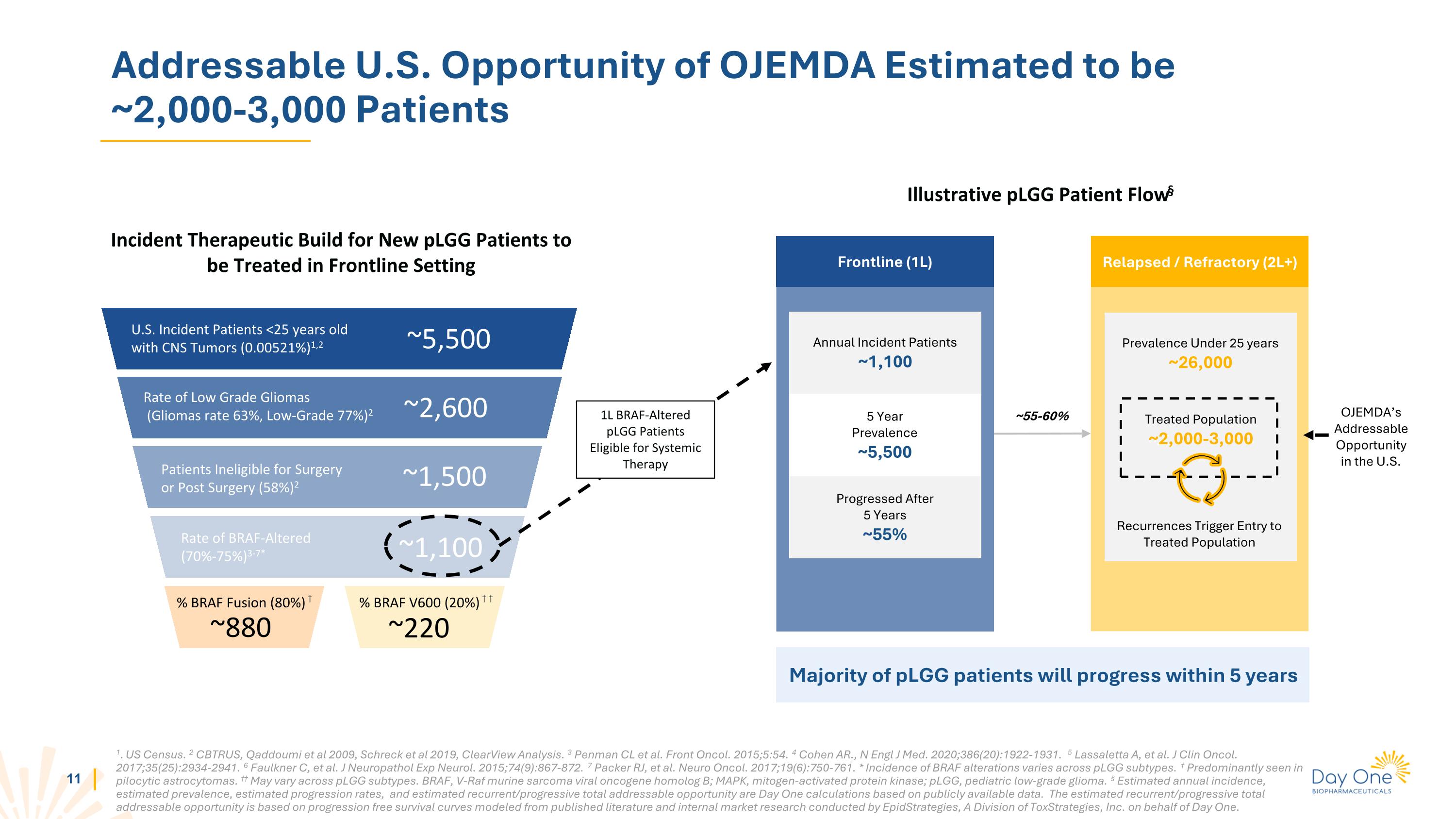
Addressable U.S. Opportunity of OJEMDA Estimated to be ~2,000-3,000 Patients Incident Therapeutic Build for New pLGG Patients to be Treated in Frontline Setting U.S. Incident Patients <25 years old with CNS Tumors (0.00521%)1,2 ~5,500 Rate of Low Grade Gliomas (Gliomas rate 63%, Low-Grade 77%)2 ~2,600 ~1,500 Patients Ineligible for Surgery or Post Surgery (58%)2 ~1,100 % BRAF Fusion (80%) † % BRAF V600 (20%) † † ~880 ~220 Frontline (1L) Annual Incident Patients ~1,100 1L BRAF-Altered pLGG Patients Eligible for Systemic Therapy Illustrative pLGG Patient Flow§ 5 Year Prevalence ~5,500 Progressed After 5 Years ~55% Relapsed / Refractory (2L+) ~55-60% 1. US Census. 2 CBTRUS, Qaddoumi et al 2009, Schreck et al 2019, ClearView Analysis. 3 Penman CL et al. Front Oncol. 2015;5:54. 4 Cohen AR., N Engl J Med. 2020;386(20):1922-1931. 5 Lassaletta A, et al. J Clin Oncol. 2017;35(25):2934-2941. 6 Faulkner C, et al. J Neuropathol Exp Neurol. 2015;74(9):867-872. 7 Packer RJ, et al. Neuro Oncol. 2017;19(6):750-761. * Incidence of BRAF alterations varies across pLGG subtypes. † Predominantly seen in pilocytic astrocytomas. †† May vary across pLGG subtypes. BRAF, V-Raf murine sarcoma viral oncogene homolog B; MAPK, mitogen-activated protein kinase; pLGG, pediatric low-grade glioma. § Estimated annual incidence, estimated prevalence, estimated progression rates, and estimated recurrent/progressive total addressable opportunity are Day One calculations based on publicly available data. The estimated recurrent/progressive total addressable opportunity is based on progression free survival curves modeled from published literature and internal market research conducted by EpidStrategies, A Division of ToxStrategies, Inc. on behalf of Day One. Majority of pLGG patients will progress within 5 years OJEMDA’s Addressable Opportunity in the U.S. Rate of BRAF-Altered (70%-75%)3-7* Prevalence Under 25 years ~26,000 Treated Population ~2,000-3,000 Recurrences Trigger Entry to Treated Population
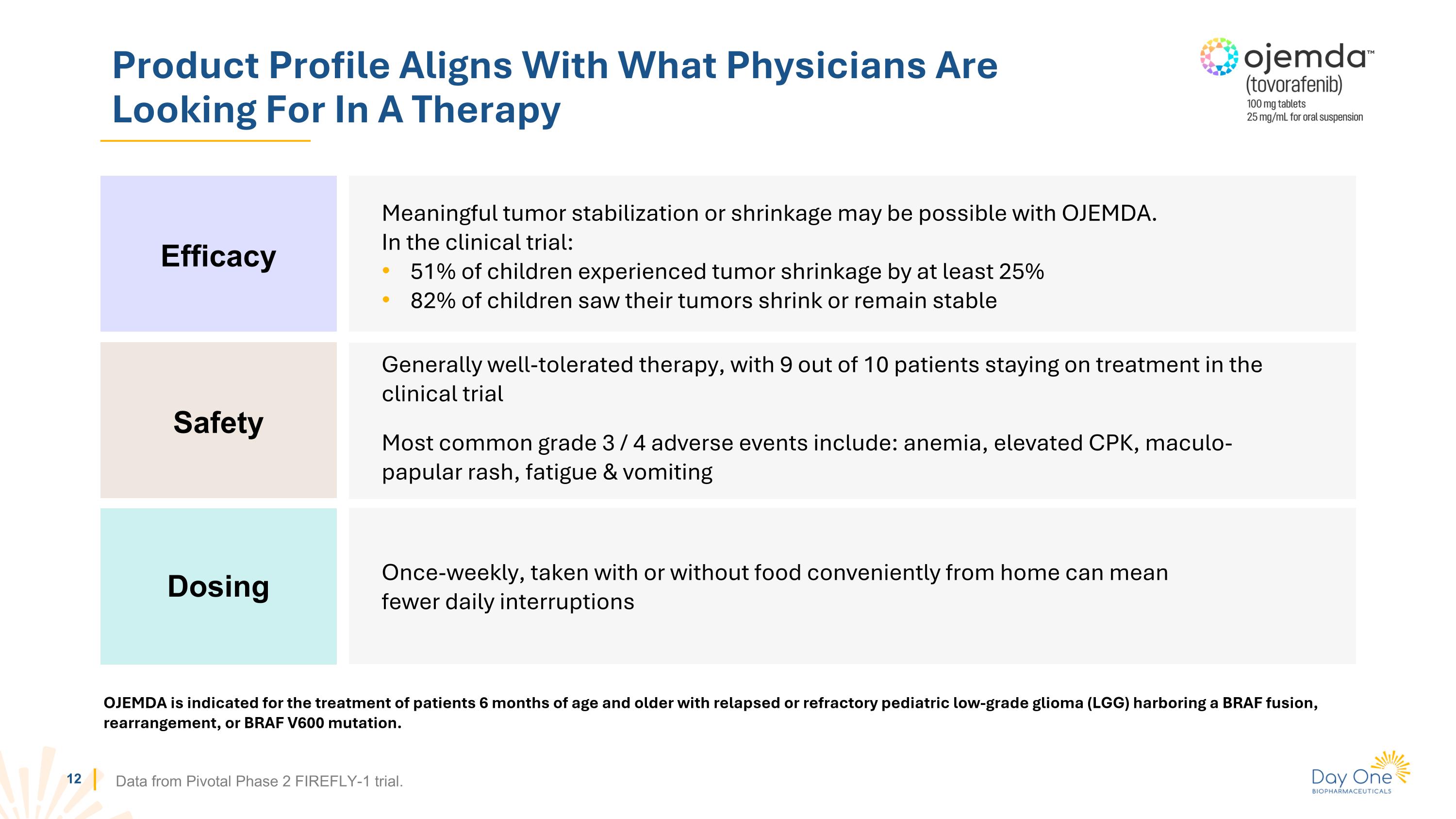
Product Profile Aligns With What Physicians Are Looking For In A Therapy Data from Pivotal Phase 2 FIREFLY-1 trial. Meaningful tumor stabilization or shrinkage may be possible with OJEMDA. In the clinical trial: 51% of children experienced tumor shrinkage by at least 25% 82% of children saw their tumors shrink or remain stable Efficacy Safety Dosing Generally well-tolerated therapy, with 9 out of 10 patients staying on treatment in the clinical trial Most common grade 3 / 4 adverse events include: anemia, elevated CPK, maculo-papular rash, fatigue & vomiting Once-weekly, taken with or without food conveniently from home can mean fewer daily interruptions OJEMDA is indicated for the treatment of patients 6 months of age and older with relapsed or refractory pediatric low-grade glioma (LGG) harboring a BRAF fusion, rearrangement, or BRAF V600 mutation.
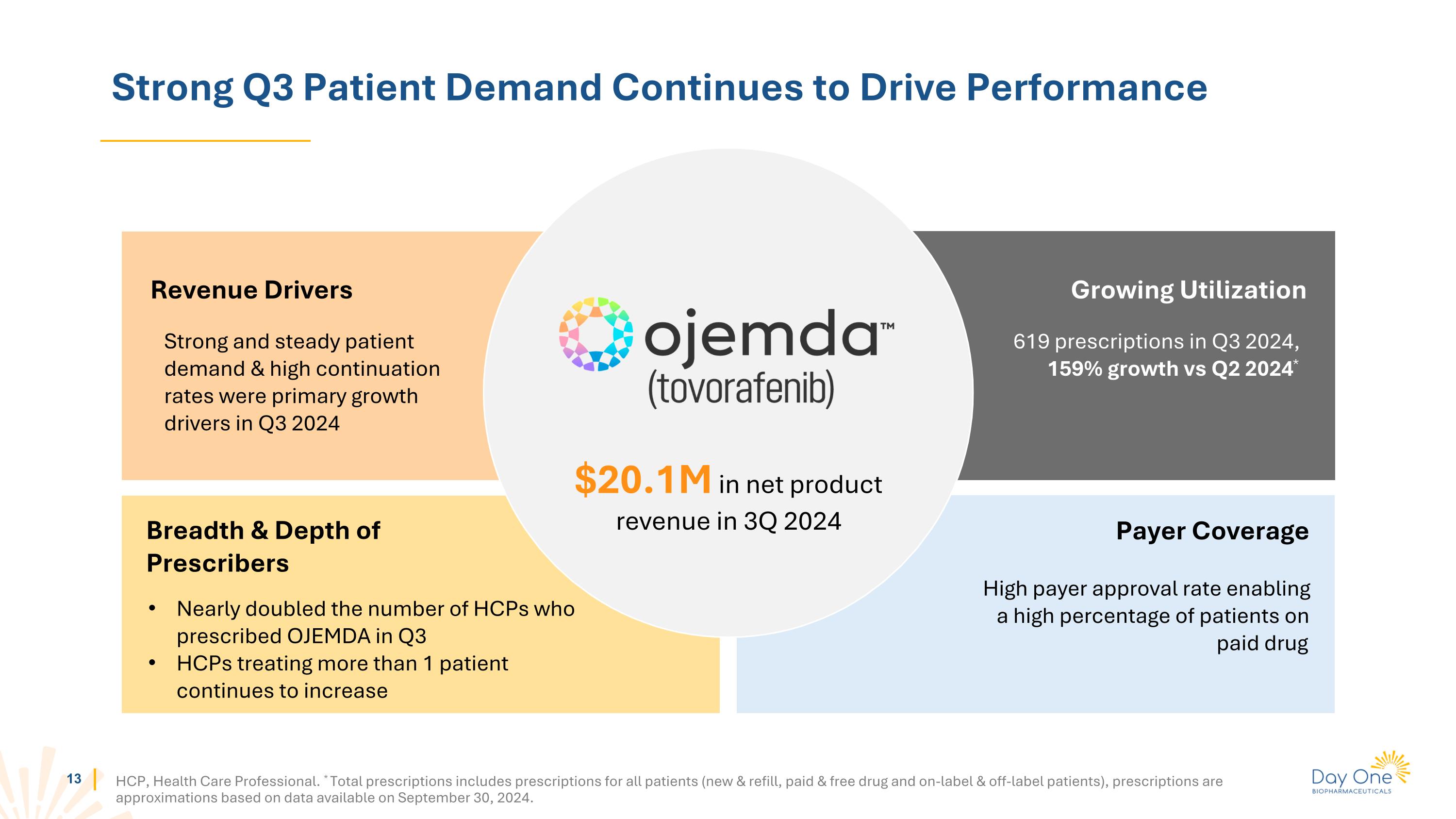
Strong Q3 Patient Demand Continues to Drive Performance HCP, Health Care Professional. * Total prescriptions includes prescriptions for all patients (new & refill, paid & free drug and on-label & off-label patients), prescriptions are approximations based on data available on September 30, 2024. Strong and steady patient demand & high continuation rates were primary growth drivers in Q3 2024 Revenue Drivers Breadth & Depth of Prescribers Growing Utilization High payer approval rate enabling a high percentage of patients on paid drug Payer Coverage $20.1M in net product revenue in 3Q 2024 619 prescriptions in Q3 2024, 159% growth vs Q2 2024* Nearly doubled the number of HCPs who prescribed OJEMDA in Q3 HCPs treating more than 1 patient continues to increase
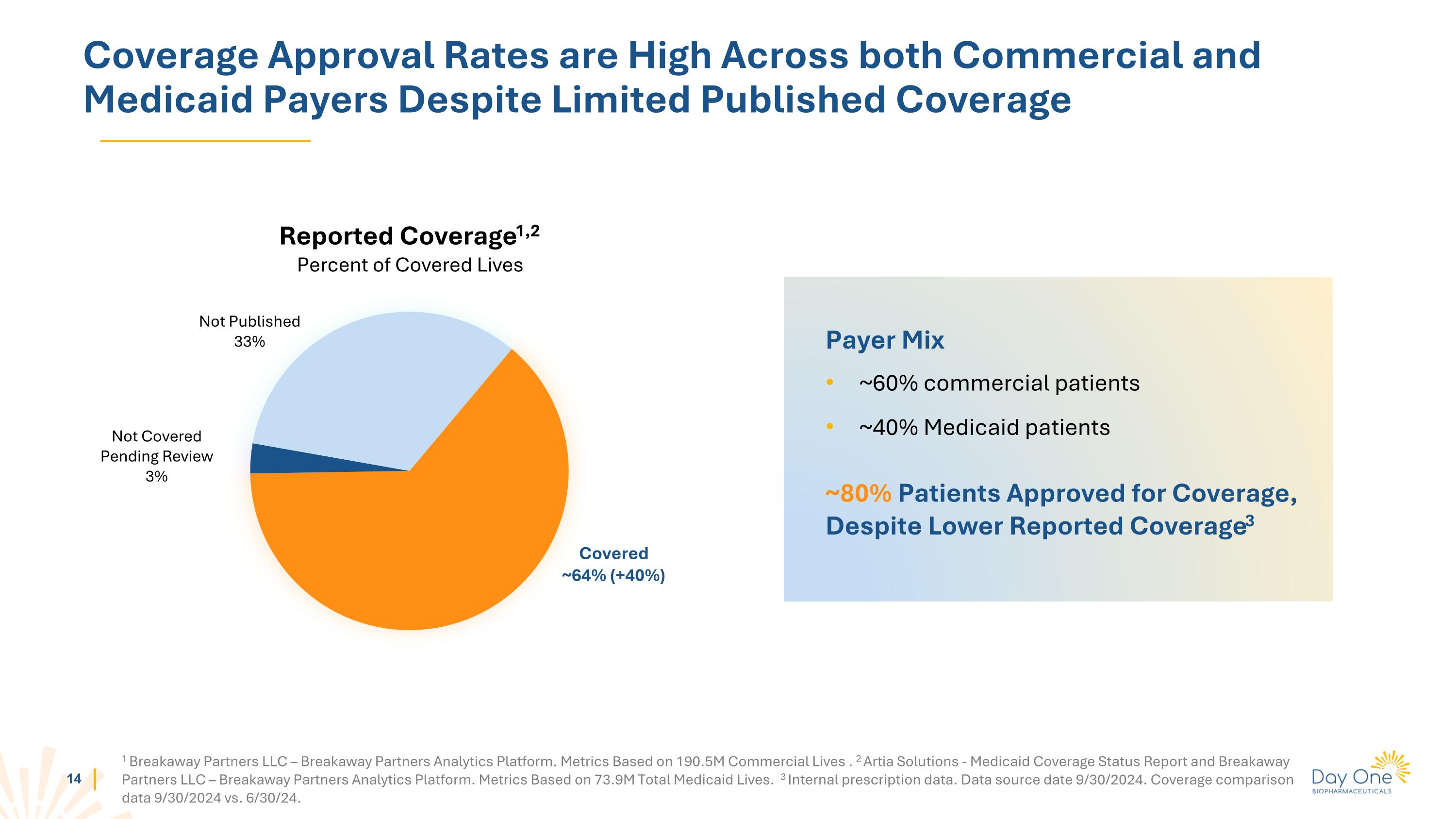
Coverage Approval Rates are High Across both Commercial and Medicaid Payers Despite Limited Published Coverage Reported Coverage1,2 Percent of Covered Lives 1 Breakaway Partners LLC – Breakaway Partners Analytics Platform. Metrics Based on 190.5M Commercial Lives . 2 Artia Solutions - Medicaid Coverage Status Report and Breakaway Partners LLC – Breakaway Partners Analytics Platform. Metrics Based on 73.9M Total Medicaid Lives. 3 Internal prescription data. Data source date 9/30/2024. Coverage comparison data 9/30/2024 vs. 6/30/24. Covered ~64% (+40%) Not Covered Pending Review 3% Not Published 33% Payer Mix ~60% commercial patients ~40% Medicaid patients ~80% Patients Approved for Coverage, Despite Lower Reported Coverage3
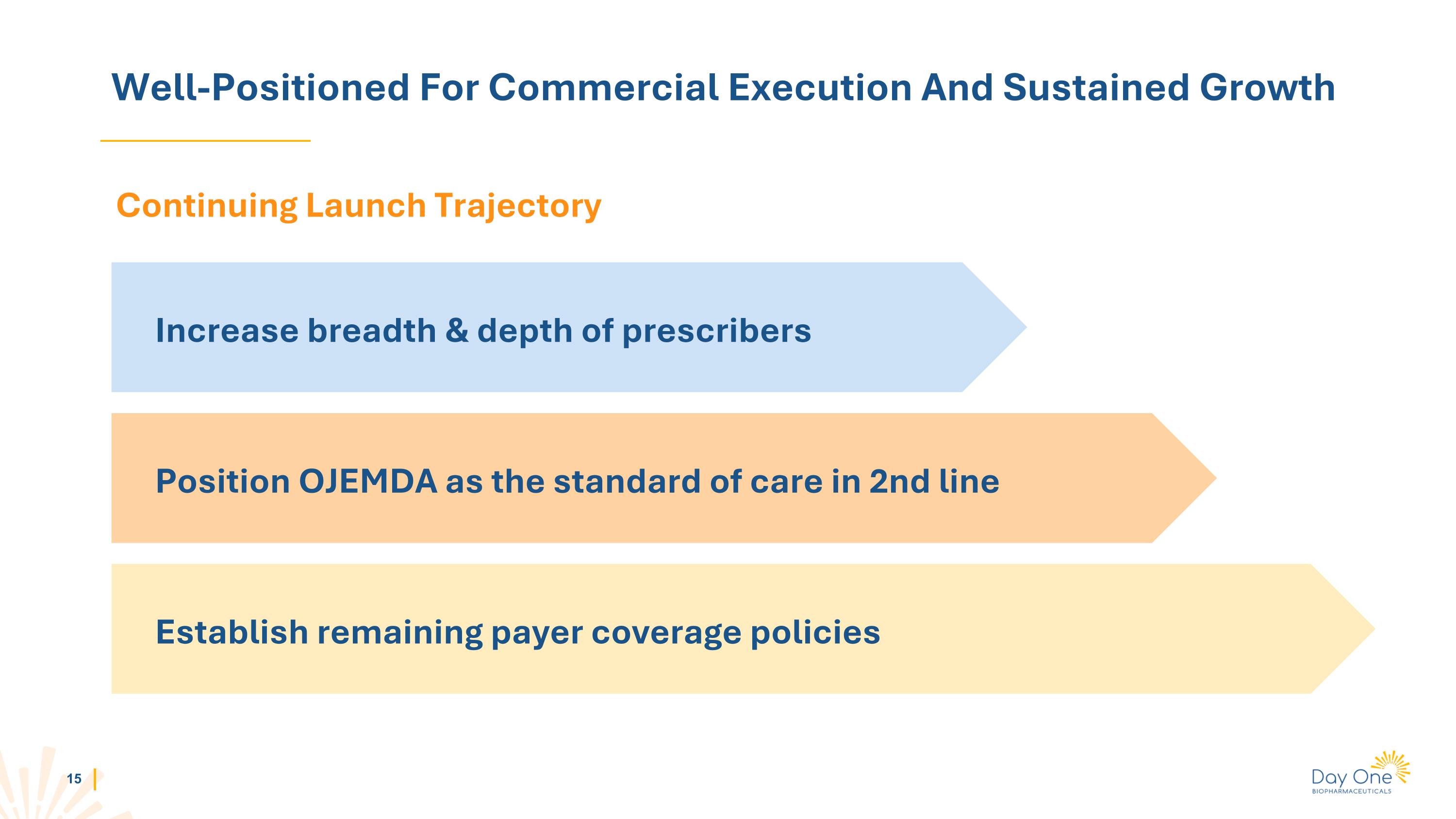
Well-Positioned For Commercial Execution And Sustained Growth Continuing Launch Trajectory Increase breadth & depth of prescribers Position OJEMDA as the standard of care in 2nd line Establish remaining payer coverage policies

FIREFLY-2 / LOGGIC Pivotal Phase 3 Trial of Tovorafenib in Frontline pLGG
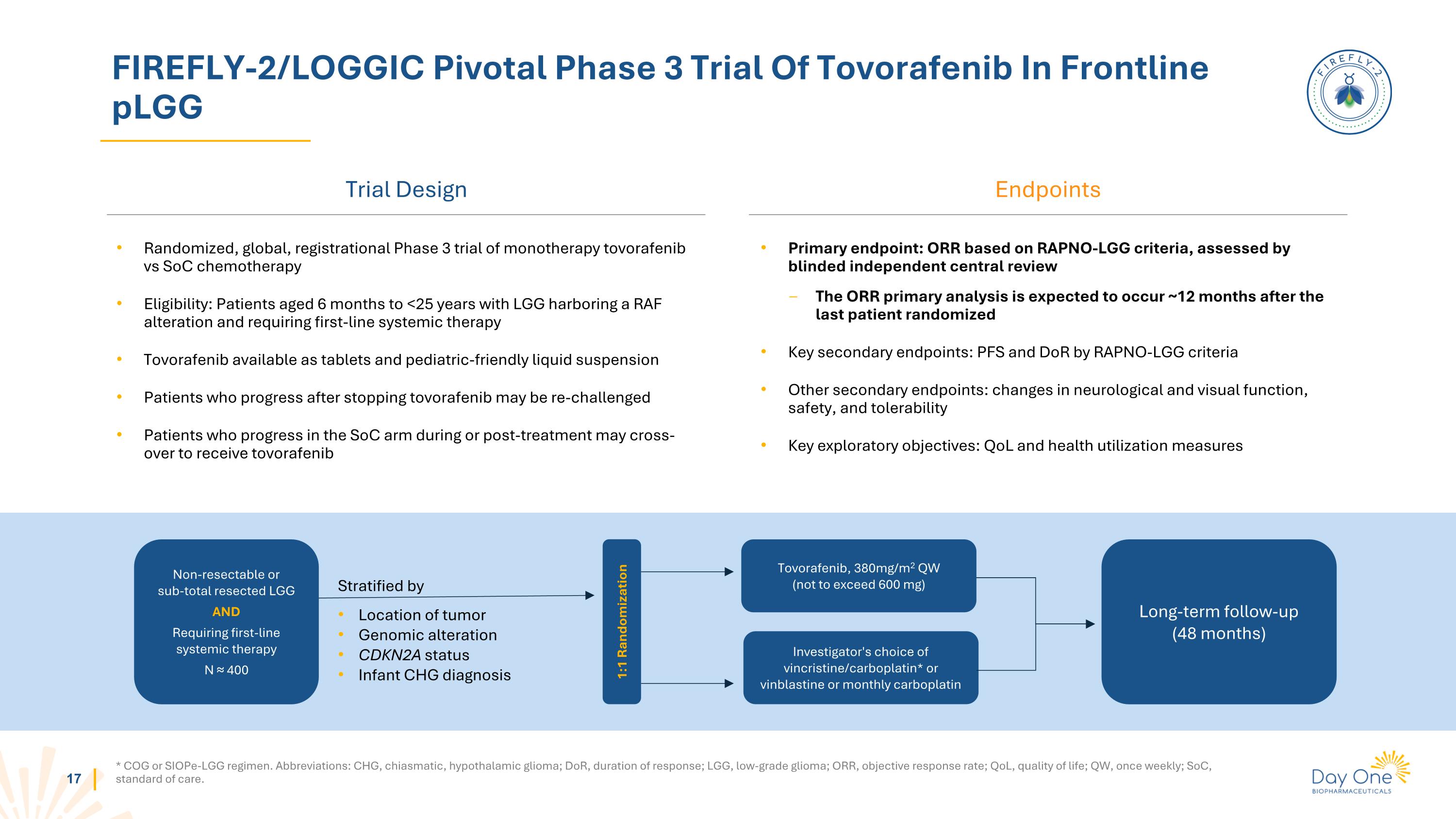
FIREFLY-2/LOGGIC Pivotal Phase 3 Trial Of Tovorafenib In Frontline pLGG Trial Design * COG or SIOPe-LGG regimen. Abbreviations: CHG, chiasmatic, hypothalamic glioma; DoR, duration of response; LGG, low-grade glioma; ORR, objective response rate; QoL, quality of life; QW, once weekly; SoC, standard of care. Endpoints Randomized, global, registrational Phase 3 trial of monotherapy tovorafenib vs SoC chemotherapy Eligibility: Patients aged 6 months to <25 years with LGG harboring a RAF alteration and requiring first-line systemic therapy Tovorafenib available as tablets and pediatric-friendly liquid suspension Patients who progress after stopping tovorafenib may be re-challenged Patients who progress in the SoC arm during or post-treatment may cross-over to receive tovorafenib Primary endpoint: ORR based on RAPNO-LGG criteria, assessed by blinded independent central review The ORR primary analysis is expected to occur ~12 months after the last patient randomized Key secondary endpoints: PFS and DoR by RAPNO-LGG criteria Other secondary endpoints: changes in neurological and visual function, safety, and tolerability Key exploratory objectives: QoL and health utilization measures Non-resectable or sub-total resected LGG AND Requiring first-line systemic therapy N ≈ 400 Stratified by Location of tumor Genomic alteration CDKN2A status Infant CHG diagnosis Tovorafenib, 380mg/m2 QW (not to exceed 600 mg) Investigator's choice of vincristine/carboplatin* or vinblastine or monthly carboplatin Long-term follow-up (48 months) 1:1 Randomization

DAY301 PTK7 Targeted Antibody Drug Conjugate (ADC)
DAY301: Next Generation ADC Targeting PTK7 1 Cho BC, et al. Ann Oncol. (34; Suppl 2): S460-S461, 2023. PTK7: Clinically-Validated ADC Target DAY301: Potential First-in-Class Asset Substantial Development and Commercial Opportunities for DAY301 Anti-tumor activity of anti-PTK7 ADC demonstrated in Phase 1b trial of Pfizer / Abbvie’s cofetuzumab pelidotin1 High PTK7 expression in multiple adult and pediatric tumor histologies U.S. IND Cleared – Target First Patient Dosed in Q4 2024 / Q1 2025 Novel ADC active in preclinical models, designed to maximize therapeutic window
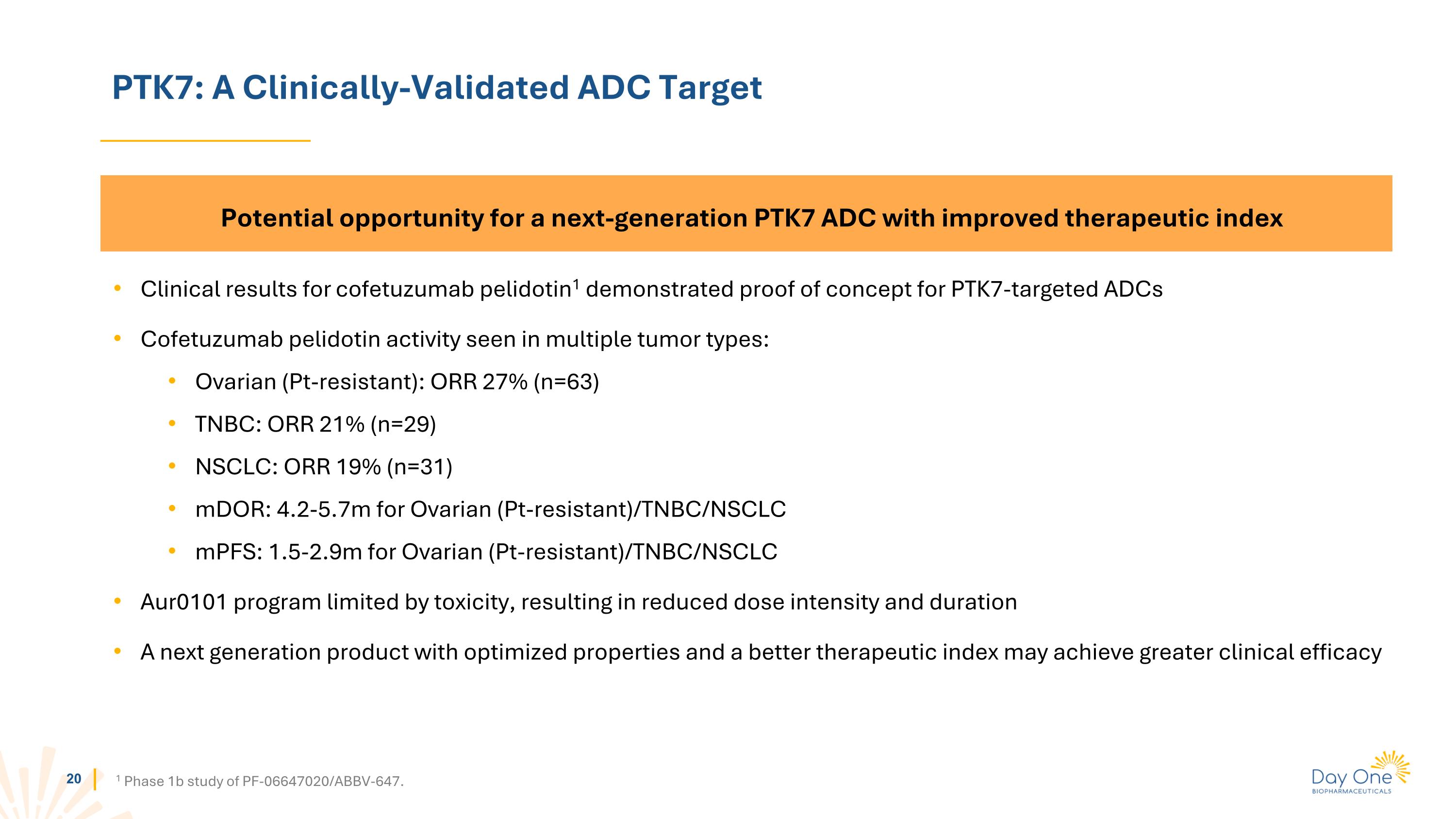
PTK7: A Clinically-Validated ADC Target 1 Phase 1b study of PF-06647020/ABBV-647. Potential opportunity for a next-generation PTK7 ADC with improved therapeutic index Clinical results for cofetuzumab pelidotin1 demonstrated proof of concept for PTK7-targeted ADCs Cofetuzumab pelidotin activity seen in multiple tumor types: Ovarian (Pt-resistant): ORR 27% (n=63) TNBC: ORR 21% (n=29) NSCLC: ORR 19% (n=31) mDOR: 4.2-5.7m for Ovarian (Pt-resistant)/TNBC/NSCLC mPFS: 1.5-2.9m for Ovarian (Pt-resistant)/TNBC/NSCLC Aur0101 program limited by toxicity, resulting in reduced dose intensity and duration A next generation product with optimized properties and a better therapeutic index may achieve greater clinical efficacy
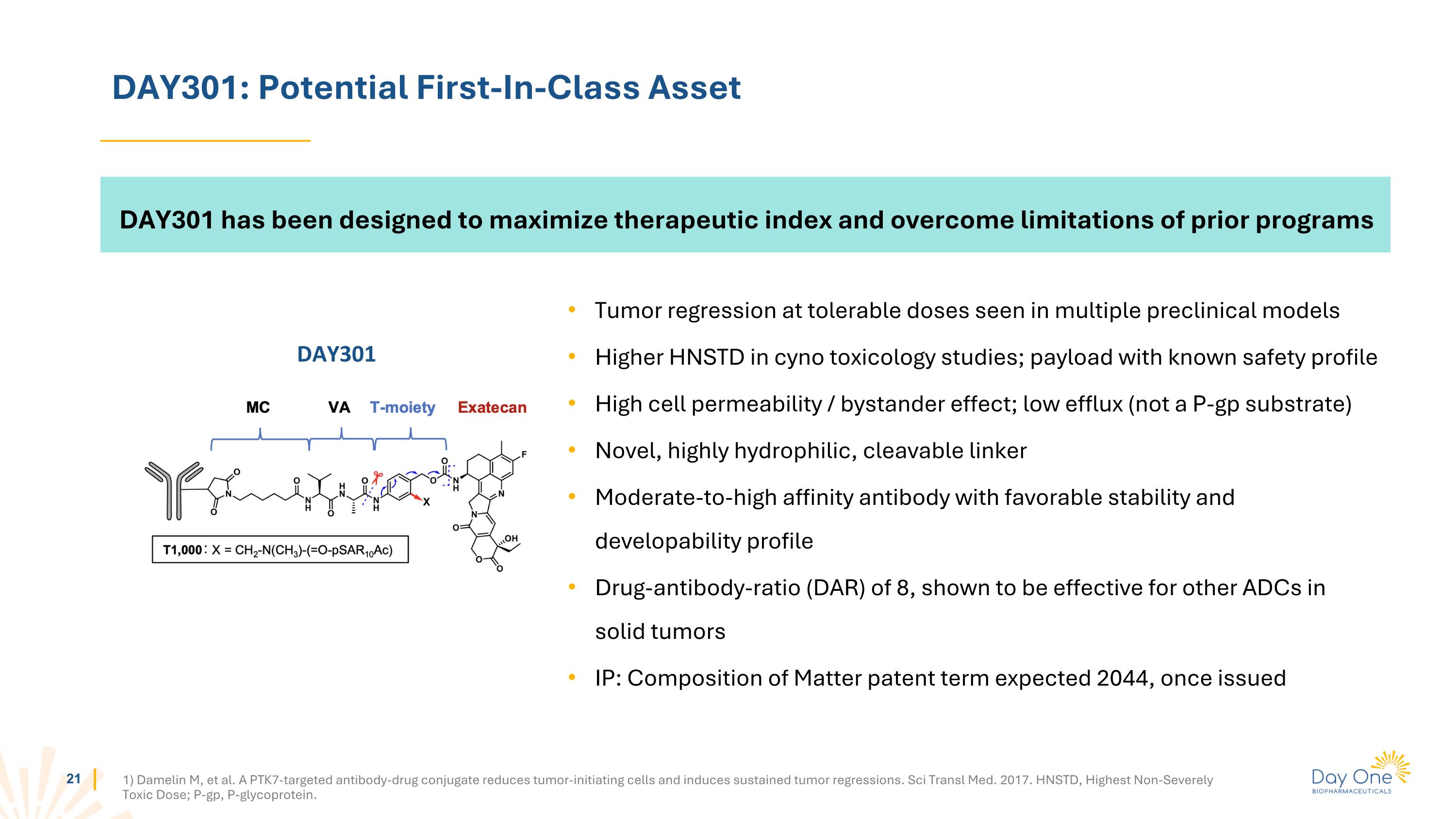
DAY301: Potential First-In-Class Asset 1) Damelin M, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017. HNSTD, Highest Non-Severely Toxic Dose; P-gp, P-glycoprotein. DAY301 has been designed to maximize therapeutic index and overcome limitations of prior programs Tumor regression at tolerable doses seen in multiple preclinical models Higher HNSTD in cyno toxicology studies; payload with known safety profile High cell permeability / bystander effect; low efflux (not a P-gp substrate) Novel, highly hydrophilic, cleavable linker Moderate-to-high affinity antibody with favorable stability and developability profile Drug-antibody-ratio (DAR) of 8, shown to be effective for other ADCs in solid tumors IP: Composition of Matter patent term expected 2044, once issued DAY301
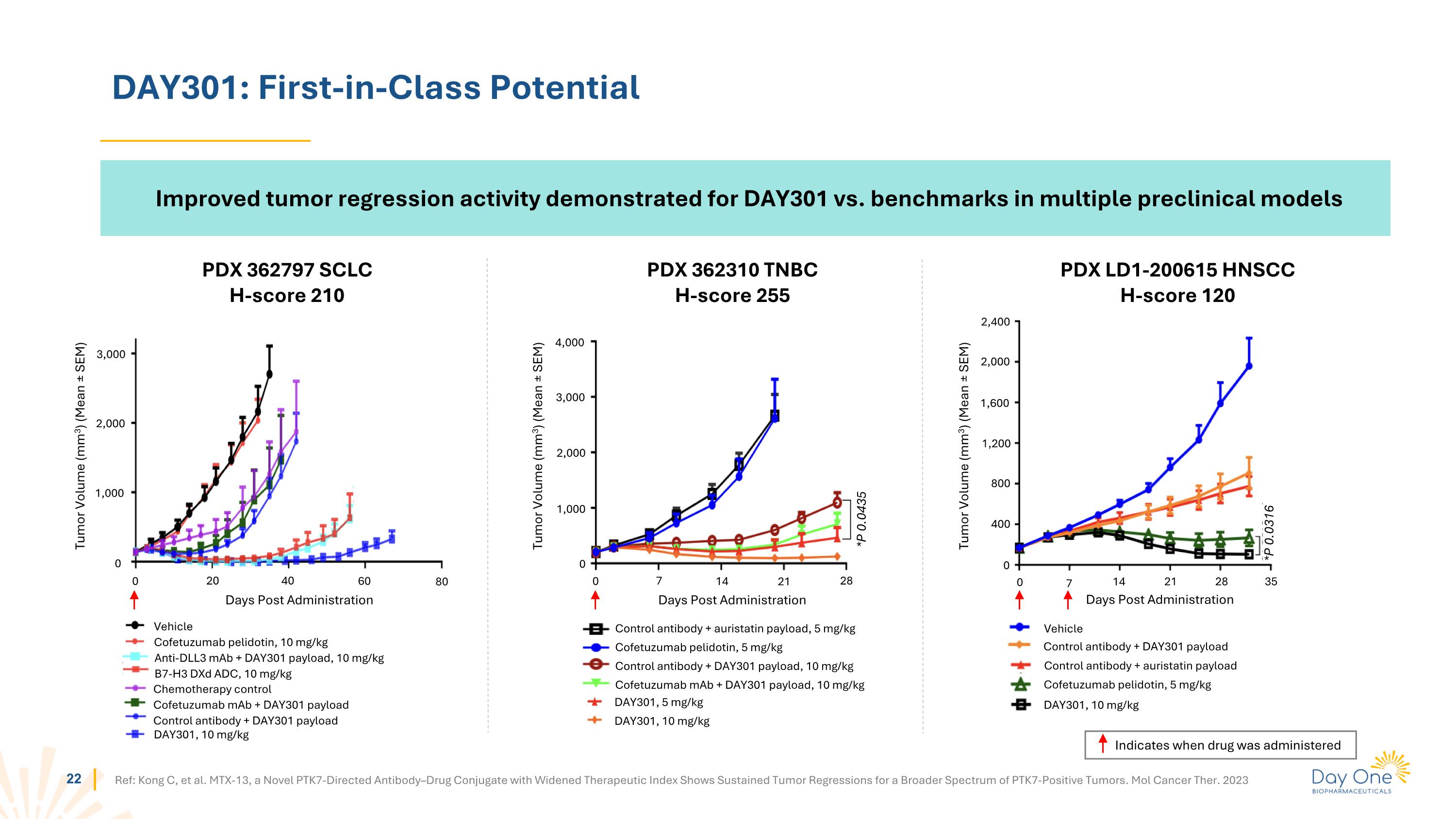
DAY301: First-in-Class Potential PDX 362310 TNBC H-score 255 PDX 362797 SCLC H-score 210 PDX LD1-200615 HNSCC H-score 120 0 1,000 2,000 3,000 0 20 40 60 80 Tumor Volume (mm3) (Mean ± SEM) Days Post Administration 0 1,000 2,000 3,000 4,000 0 7 14 21 28 Tumor Volume (mm3) (Mean ± SEM) Days Post Administration *P 0.0435 0 400 800 1,200 1,600 2,000 2,400 0 7 14 21 28 35 *P 0.0316 Tumor Volume (mm3) (Mean ± SEM) Days Post Administration Control antibody + DAY301 payload Vehicle Control antibody + auristatin payload Cofetuzumab pelidotin, 5 mg/kg DAY301, 10 mg/kg Ref: Kong C, et al. MTX-13, a Novel PTK7-Directed Antibody–Drug Conjugate with Widened Therapeutic Index Shows Sustained Tumor Regressions for a Broader Spectrum of PTK7-Positive Tumors. Mol Cancer Ther. 2023 Control antibody + auristatin payload, 5 mg/kg Cofetuzumab pelidotin, 5 mg/kg Control antibody + DAY301 payload, 10 mg/kg Cofetuzumab mAb + DAY301 payload, 10 mg/kg DAY301, 5 mg/kg DAY301, 10 mg/kg Vehicle Cofetuzumab pelidotin, 10 mg/kg Chemotherapy control Cofetuzumab mAb + DAY301 payload Control antibody + DAY301 payload Anti-DLL3 mAb + DAY301 payload, 10 mg/kg B7-H3 DXd ADC, 10 mg/kg DAY301, 10 mg/kg Indicates when drug was administered Improved tumor regression activity demonstrated for DAY301 vs. benchmarks in multiple preclinical models
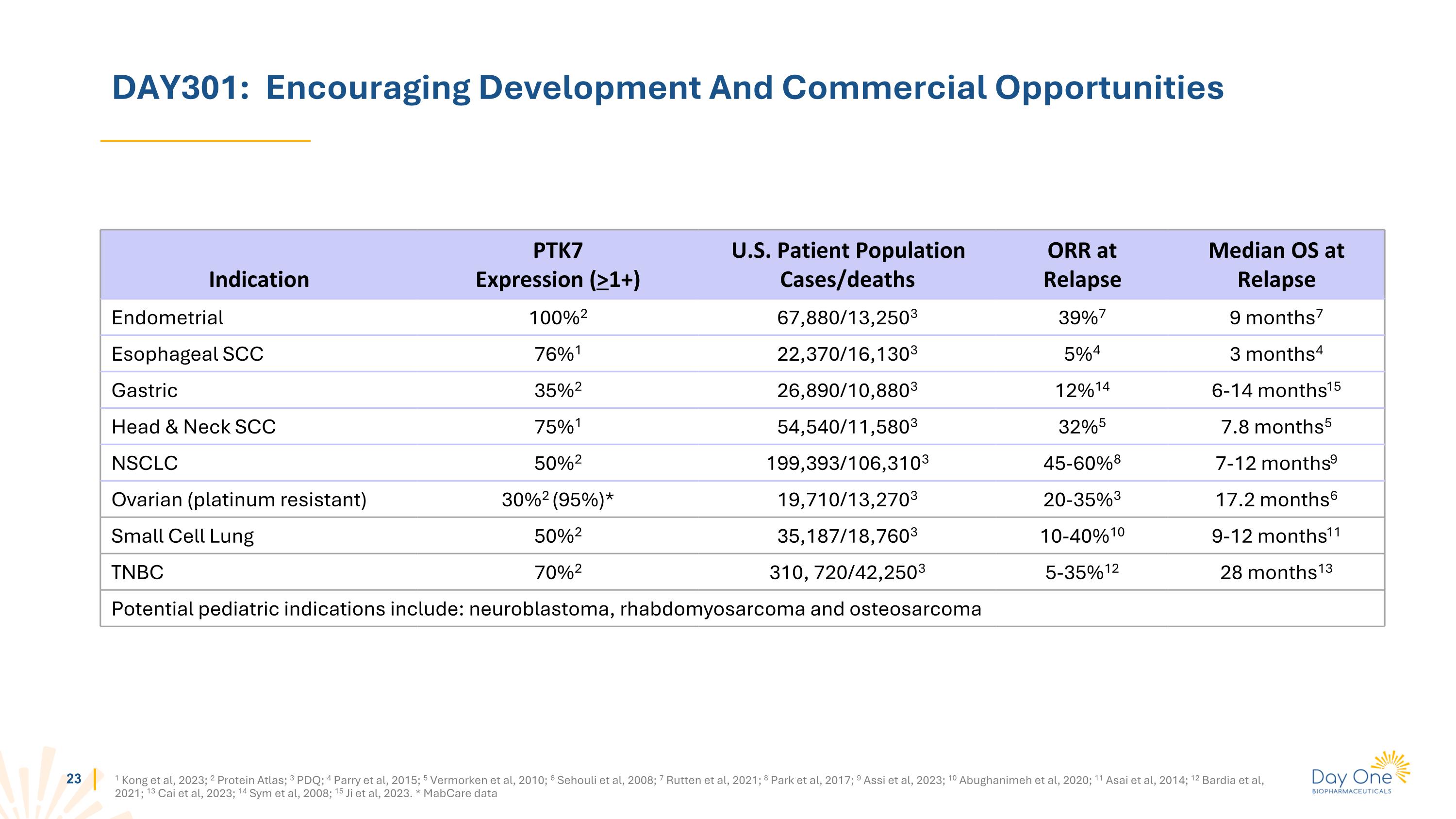
DAY301: Encouraging Development And Commercial Opportunities Indication PTK7 Expression (>1+) U.S. Patient Population Cases/deaths ORR at Relapse Median OS at Relapse Endometrial 100%2 67,880/13,2503 39%7 9 months7 Esophageal SCC 76%1 22,370/16,1303 5%4 3 months4 Gastric 35%2 26,890/10,8803 12%14 6-14 months15 Head & Neck SCC 75%1 54,540/11,5803 32%5 7.8 months5 NSCLC 50%2 199,393/106,3103 45-60%8 7-12 months9 Ovarian (platinum resistant) 30%2 (95%)* 19,710/13,2703 20-35%3 17.2 months6 Small Cell Lung 50%2 35,187/18,7603 10-40%10 9-12 months11 TNBC 70%2 310, 720/42,2503 5-35%12 28 months13 Potential pediatric indications include: neuroblastoma, rhabdomyosarcoma and osteosarcoma 1 Kong et al, 2023; 2 Protein Atlas; 3 PDQ; 4 Parry et al, 2015; 5 Vermorken et al, 2010; 6 Sehouli et al, 2008; 7 Rutten et al, 2021; 8 Park et al, 2017; 9 Assi et al, 2023; 10 Abughanimeh et al, 2020; 11 Asai et al, 2014; 12 Bardia et al, 2021; 13 Cai et al, 2023; 14 Sym et al, 2008; 15 Ji et al, 2023. * MabCare data
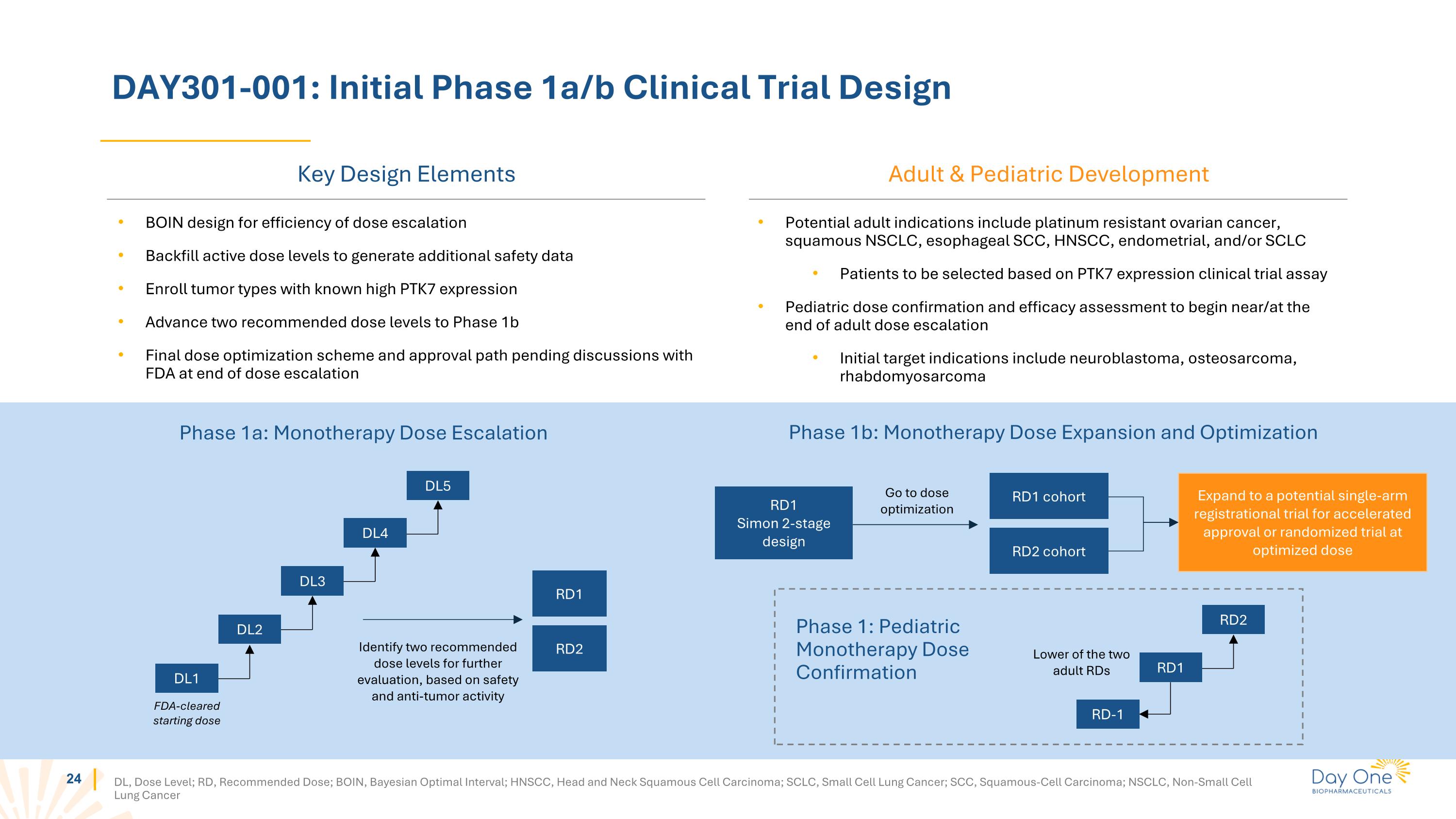
DAY301-001: Initial Phase 1a/b Clinical Trial Design DL, Dose Level; RD, Recommended Dose; BOIN, Bayesian Optimal Interval; HNSCC, Head and Neck Squamous Cell Carcinoma; SCLC, Small Cell Lung Cancer; SCC, Squamous-Cell Carcinoma; NSCLC, Non-Small Cell Lung Cancer Phase 1a: Monotherapy Dose Escalation FDA-cleared starting dose DL5 RD1 RD2 Identify two recommended dose levels for further evaluation, based on safety and anti-tumor activity BOIN design for efficiency of dose escalation Backfill active dose levels to generate additional safety data Enroll tumor types with known high PTK7 expression Advance two recommended dose levels to Phase 1b Final dose optimization scheme and approval path pending discussions with FDA at end of dose escalation RD1 Simon 2-stage design Expand to a potential single-arm registrational trial for accelerated approval or randomized trial at optimized dose RD1 cohort RD2 cohort Go to dose optimization Phase 1b: Monotherapy Dose Expansion and Optimization Phase 1: Pediatric Monotherapy Dose Confirmation RD-1 RD2 Lower of the two adult RDs Potential adult indications include platinum resistant ovarian cancer, squamous NSCLC, esophageal SCC, HNSCC, endometrial, and/or SCLC Patients to be selected based on PTK7 expression clinical trial assay Pediatric dose confirmation and efficacy assessment to begin near/at the end of adult dose escalation Initial target indications include neuroblastoma, osteosarcoma, rhabdomyosarcoma Key Design Elements Adult & Pediatric Development DL4 DL3 DL2 DL1 RD1

Summary
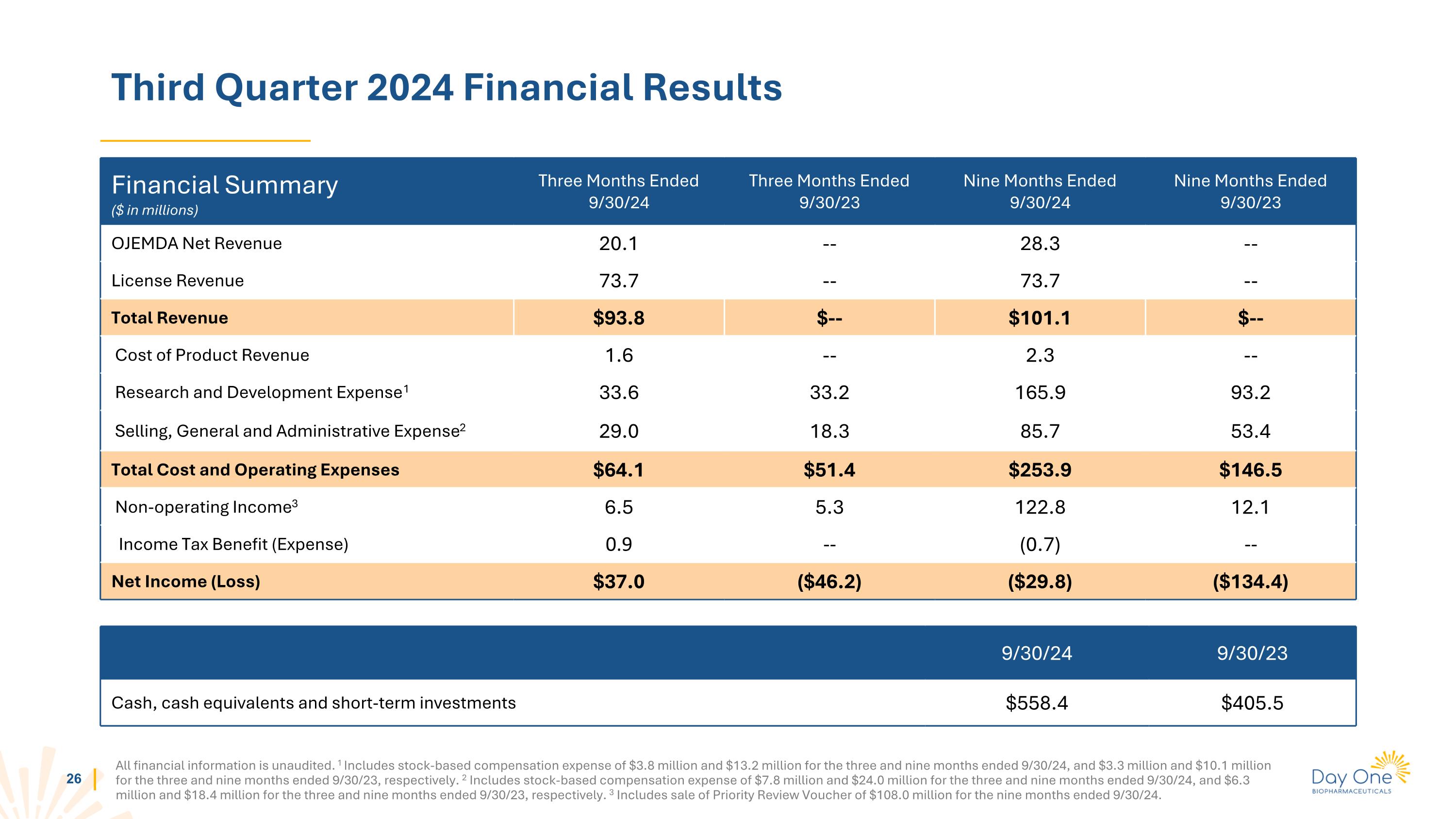
Third Quarter 2024 Financial Results All financial information is unaudited. 1 Includes stock-based compensation expense of $3.8 million and $13.2 million for the three and nine months ended 9/30/24, and $3.3 million and $10.1 million for the three and nine months ended 9/30/23, respectively. 2 Includes stock-based compensation expense of $7.8 million and $24.0 million for the three and nine months ended 9/30/24, and $6.3 million and $18.4 million for the three and nine months ended 9/30/23, respectively. 3 Includes sale of Priority Review Voucher of $108.0 million for the nine months ended 9/30/24. Financial Summary ($ in millions) Three Months Ended 9/30/24 Three Months Ended 9/30/23 Nine Months Ended 9/30/24 Nine Months Ended 9/30/23 OJEMDA Net Revenue 20.1 -- 28.3 -- License Revenue 73.7 -- 73.7 -- Total Revenue $93.8 $-- $101.1 $-- Cost of Product Revenue 1.6 -- 2.3 -- Research and Development Expense1 33.6 33.2 165.9 93.2 Selling, General and Administrative Expense2 29.0 18.3 85.7 53.4 Total Cost and Operating Expenses $64.1 $51.4 $253.9 $146.5 Non-operating Income3 6.5 5.3 122.8 12.1 Income Tax Benefit (Expense) 0.9 -- (0.7) -- Net Income (Loss) $37.0 ($46.2) ($29.8) ($134.4) 9/30/24 9/30/23 Cash, cash equivalents and short-term investments $558.4 $405.5
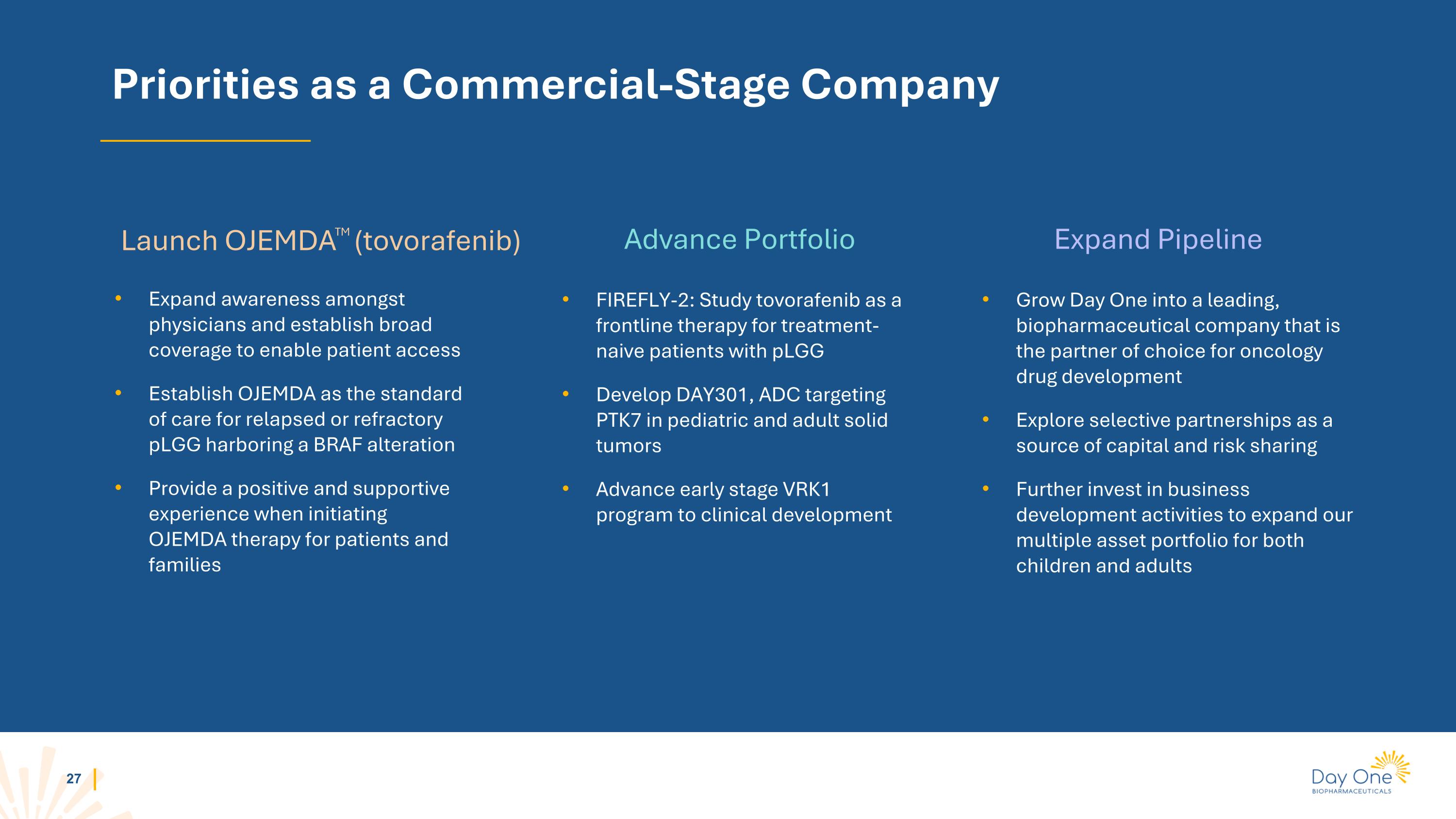
Priorities as a Commercial-Stage Company Expand awareness amongst physicians and establish broad coverage to enable patient access Establish OJEMDA as the standard of care for relapsed or refractory pLGG harboring a BRAF alteration Provide a positive and supportive experience when initiating OJEMDA therapy for patients and families Grow Day One into a leading, biopharmaceutical company that is the partner of choice for oncology drug development Explore selective partnerships as a source of capital and risk sharing Further invest in business development activities to expand our multiple asset portfolio for both children and adults FIREFLY-2: Study tovorafenib as a frontline therapy for treatment-naive patients with pLGG Develop DAY301, ADC targeting PTK7 in pediatric and adult solid tumors Advance early stage VRK1 program to clinical development Launch OJEMDATM (tovorafenib) Advance Portfolio Expand Pipeline

Appendix
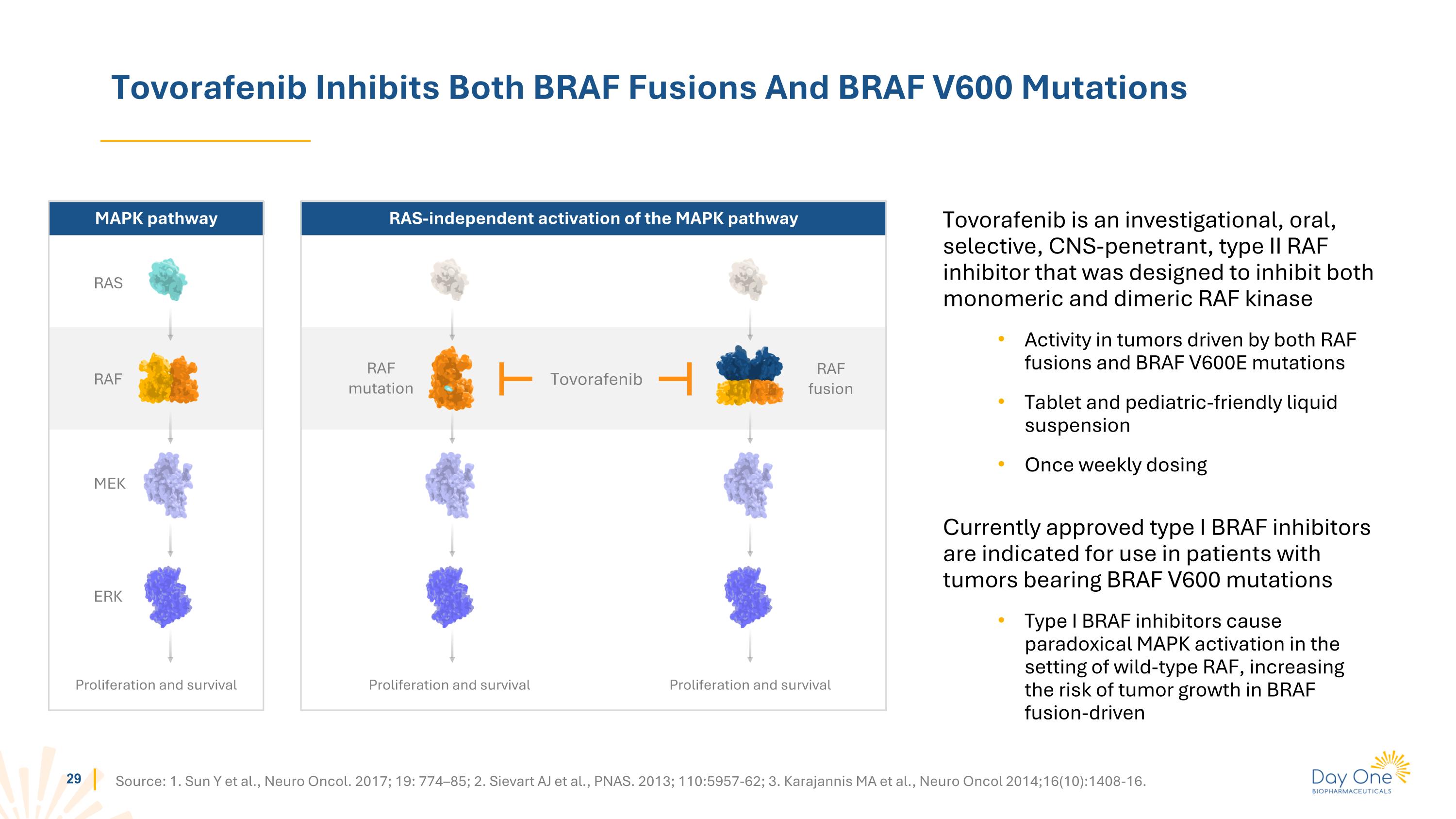
Tovorafenib Inhibits Both BRAF Fusions And BRAF V600 Mutations Tovorafenib is an investigational, oral, selective, CNS-penetrant, type II RAF inhibitor that was designed to inhibit both monomeric and dimeric RAF kinase Activity in tumors driven by both RAF fusions and BRAF V600E mutations Tablet and pediatric-friendly liquid suspension Once weekly dosing Currently approved type I BRAF inhibitors are indicated for use in patients with tumors bearing BRAF V600 mutations Type I BRAF inhibitors cause paradoxical MAPK activation in the setting of wild-type RAF, increasing the risk of tumor growth in BRAF fusion-driven Source: 1. Sun Y et al., Neuro Oncol. 2017; 19: 774–85; 2. Sievart AJ et al., PNAS. 2013; 110:5957-62; 3. Karajannis MA et al., Neuro Oncol 2014;16(10):1408-16. RAS-independent activation of the MAPK pathway MAPK pathway RAS RAF MEK ERK Proliferation and survival RAF mutation RAF fusion Proliferation and survival Proliferation and survival Tovorafenib
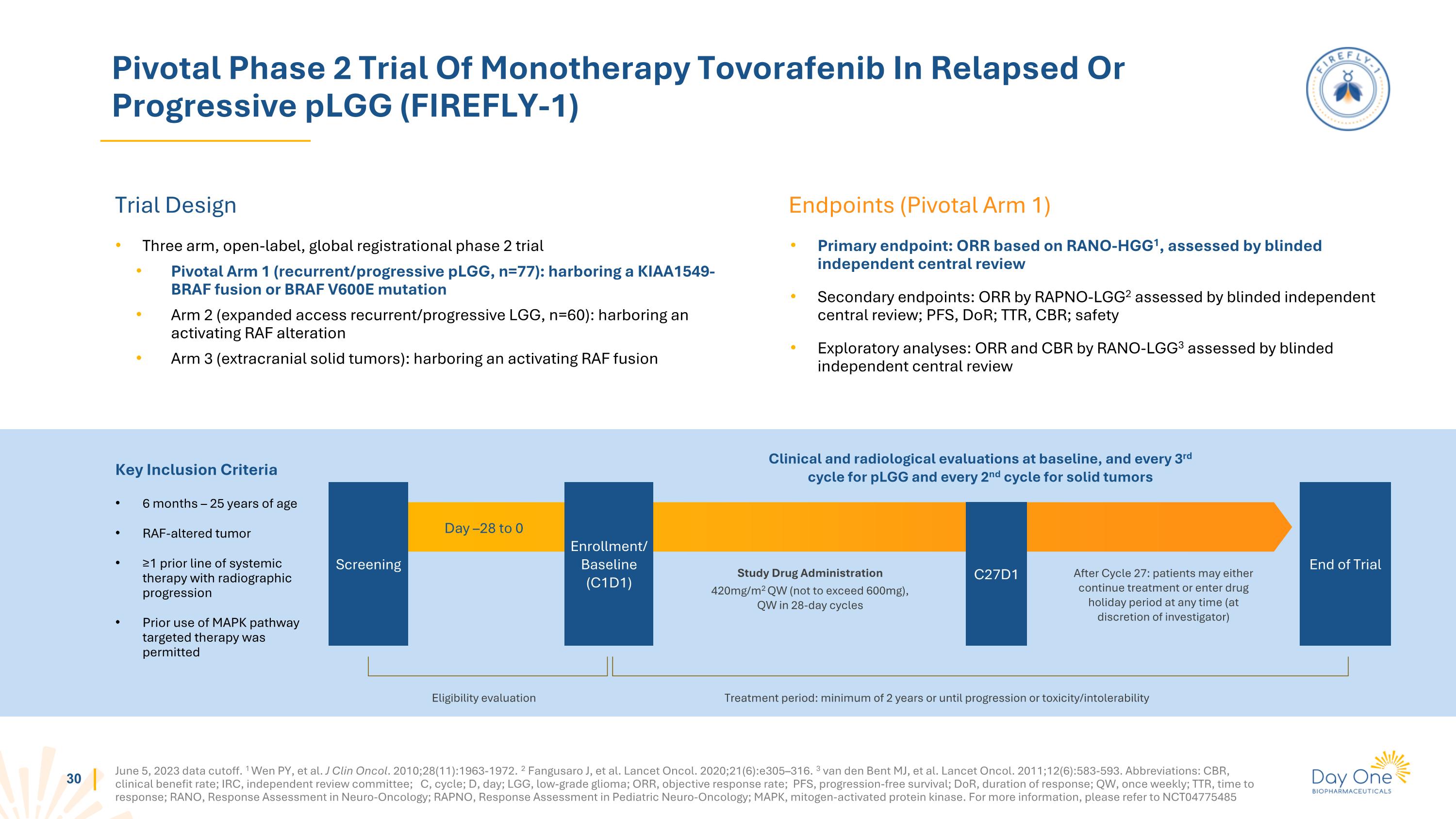
June 5, 2023 data cutoff. 1 Wen PY, et al. J Clin Oncol. 2010;28(11):1963-1972. 2 Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. 3 van den Bent MJ, et al. Lancet Oncol. 2011;12(6):583-593. Abbreviations: CBR, clinical benefit rate; IRC, independent review committee; C, cycle; D, day; LGG, low-grade glioma; ORR, objective response rate; PFS, progression-free survival; DoR, duration of response; QW, once weekly; TTR, time to response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; MAPK, mitogen-activated protein kinase. For more information, please refer to NCT04775485 Pivotal Phase 2 Trial Of Monotherapy Tovorafenib In Relapsed Or Progressive pLGG (FIREFLY-1) Trial Design Endpoints (Pivotal Arm 1) Three arm, open-label, global registrational phase 2 trial Pivotal Arm 1 (recurrent/progressive pLGG, n=77): harboring a KIAA1549-BRAF fusion or BRAF V600E mutation Arm 2 (expanded access recurrent/progressive LGG, n=60): harboring an activating RAF alteration Arm 3 (extracranial solid tumors): harboring an activating RAF fusion Primary endpoint: ORR based on RANO-HGG1, assessed by blinded independent central review Secondary endpoints: ORR by RAPNO-LGG2 assessed by blinded independent central review; PFS, DoR; TTR, CBR; safety Exploratory analyses: ORR and CBR by RANO-LGG3 assessed by blinded independent central review Day –28 to 0 Study Drug Administration 420mg/m2 QW (not to exceed 600mg), QW in 28-day cycles After Cycle 27: patients may either continue treatment or enter drug holiday period at any time (at discretion of investigator) Screening C27D1 Enrollment/ Baseline (C1D1) End of Trial Clinical and radiological evaluations at baseline, and every 3rd cycle for pLGG and every 2nd cycle for solid tumors Eligibility evaluation Treatment period: minimum of 2 years or until progression or toxicity/intolerability Key Inclusion Criteria 6 months – 25 years of age RAF-altered tumor ≥1 prior line of systemic therapy with radiographic progression Prior use of MAPK pathway targeted therapy was permitted

Data from Pivotal Phase 2 FIREFLY-1 Trial June 5, 2023 data cutoff
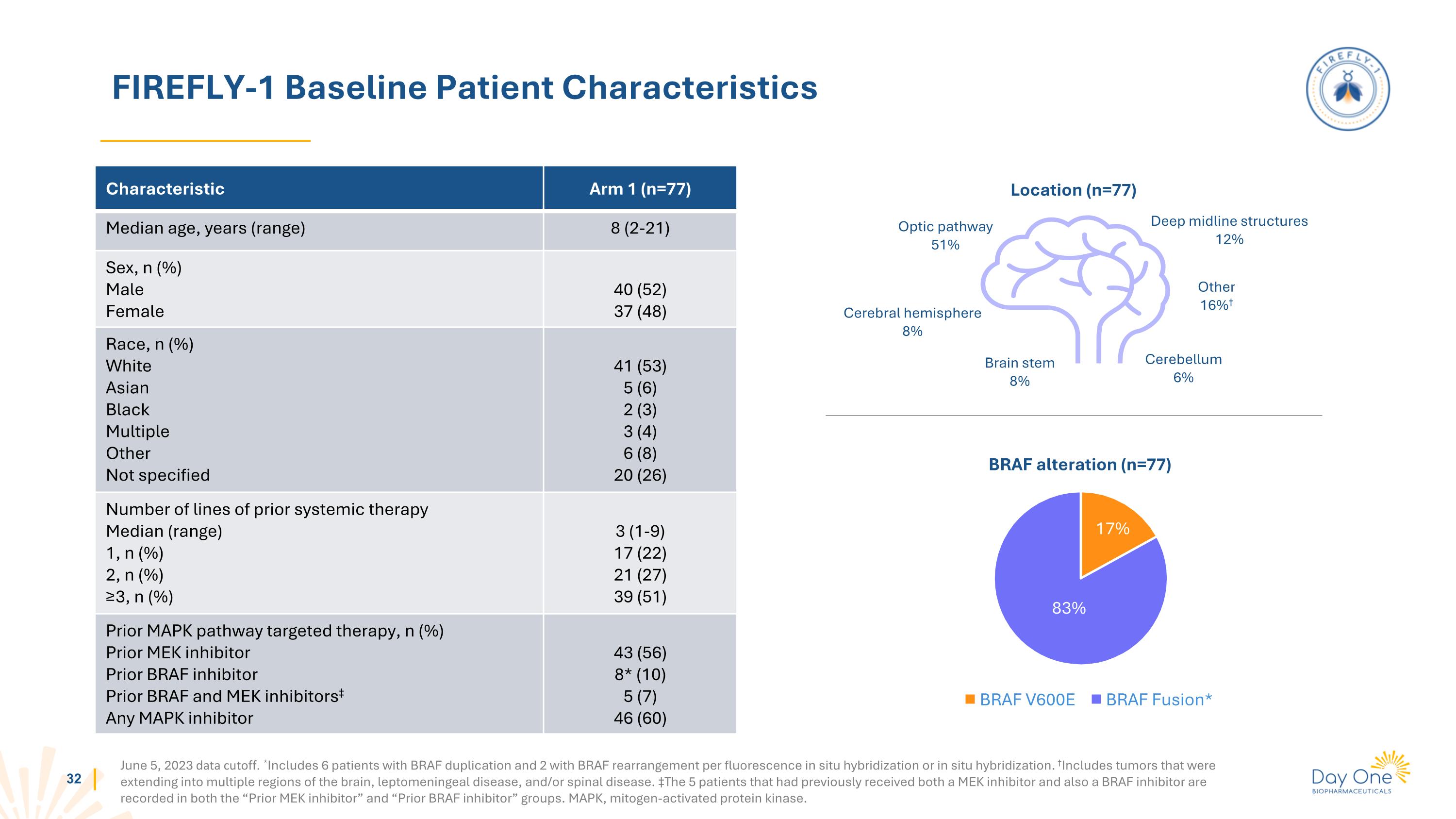
FIREFLY-1 Baseline Patient Characteristics June 5, 2023 data cutoff. *Includes 6 patients with BRAF duplication and 2 with BRAF rearrangement per fluorescence in situ hybridization or in situ hybridization. †Includes tumors that were extending into multiple regions of the brain, leptomeningeal disease, and/or spinal disease. ‡The 5 patients that had previously received both a MEK inhibitor and also a BRAF inhibitor are recorded in both the “Prior MEK inhibitor” and “Prior BRAF inhibitor” groups. MAPK, mitogen-activated protein kinase. Characteristic Arm 1 (n=77) Median age, years (range) 8 (2-21) Sex, n (%) Male Female 40 (52) 37 (48) Race, n (%) White Asian Black Multiple Other Not specified 41 (53) 5 (6) 2 (3) 3 (4) 6 (8) 20 (26) Number of lines of prior systemic therapy Median (range) 1, n (%) 2, n (%) ≥3, n (%) 3 (1-9) 17 (22) 21 (27) 39 (51) Prior MAPK pathway targeted therapy, n (%) Prior MEK inhibitor Prior BRAF inhibitor Prior BRAF and MEK inhibitors‡ Any MAPK inhibitor 43 (56) 8* (10) 5 (7) 46 (60) BRAF alteration (n=77) Location (n=77) Optic pathway 51% Cerebral hemisphere 8% Brain stem 8% Deep midline structures 12% Other 16%† Cerebellum 6% 17% 83%
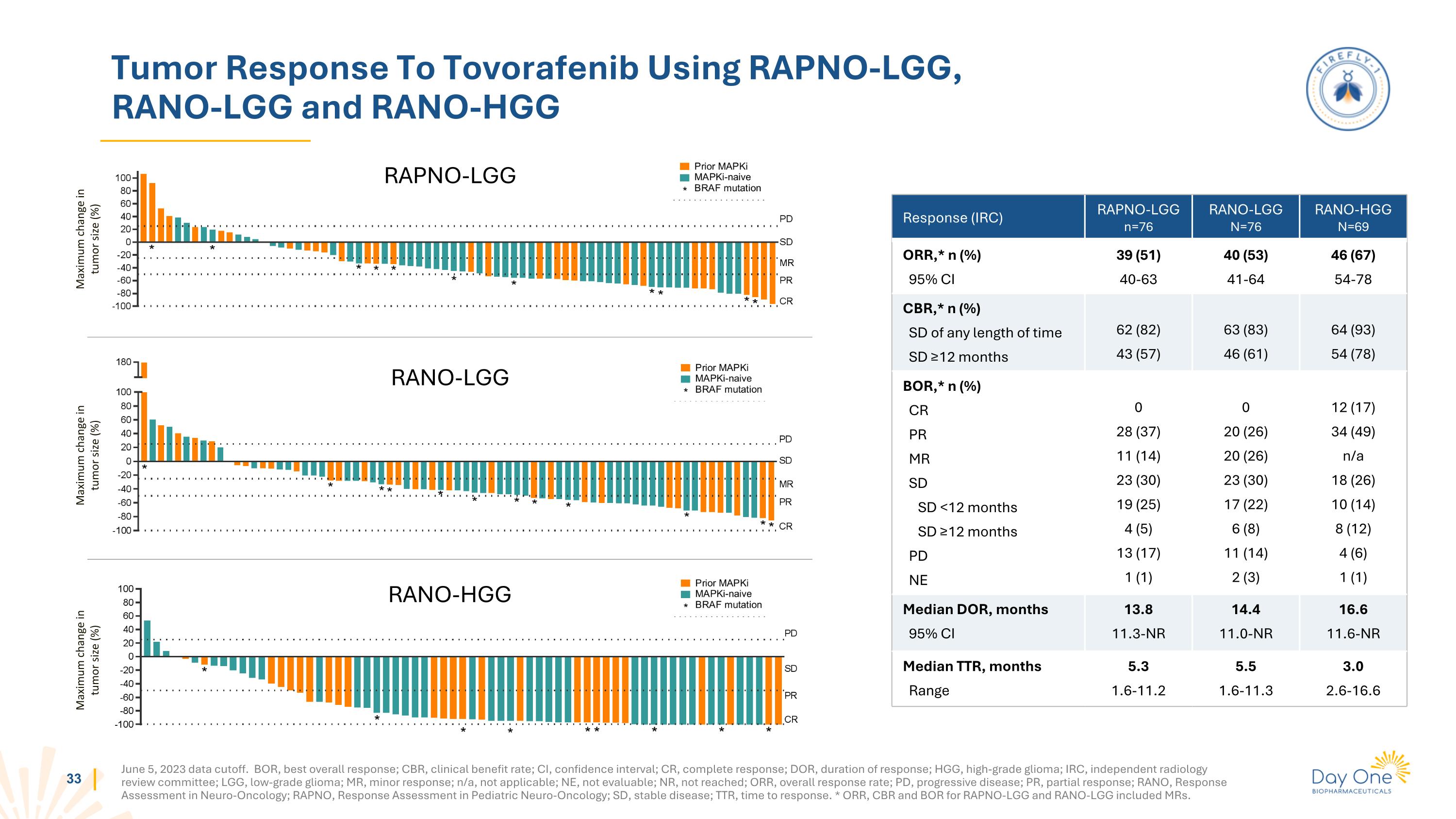
Tumor Response To Tovorafenib Using RAPNO-LGG, RANO-LGG and RANO-HGG Maximum change in tumor size (%) Maximum change in tumor size (%) Maximum change in tumor size (%) RAPNO-LGG RANO-LGG RANO-HGG June 5, 2023 data cutoff. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DOR, duration of response; HGG, high-grade glioma; IRC, independent radiology review committee; LGG, low-grade glioma; MR, minor response; n/a, not applicable; NE, not evaluable; NR, not reached; ORR, overall response rate; PD, progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RAPNO, Response Assessment in Pediatric Neuro-Oncology; SD, stable disease; TTR, time to response. * ORR, CBR and BOR for RAPNO-LGG and RANO-LGG included MRs. Response (IRC) RAPNO-LGG n=76 RANO-LGG N=76 RANO-HGG N=69 ORR,* n (%) 95% CI 39 (51) 40-63 40 (53) 41-64 46 (67) 54-78 CBR,* n (%) SD of any length of time SD ≥12 months 62 (82) 43 (57) 63 (83) 46 (61) 64 (93) 54 (78) BOR,* n (%) CR PR MR SD SD <12 months SD ≥12 months PD NE 0 28 (37) 11 (14) 23 (30) 19 (25) 4 (5) 13 (17) 1 (1) 0 20 (26) 20 (26) 23 (30) 17 (22) 6 (8) 11 (14) 2 (3) 12 (17) 34 (49) n/a 18 (26) 10 (14) 8 (12) 4 (6) 1 (1) Median DOR, months 95% CI 13.8 11.3-NR 14.4 11.0-NR 16.6 11.6-NR Median TTR, months Range 5.3 1.6-11.2 5.5 1.6-11.3 3.0 2.6-16.6
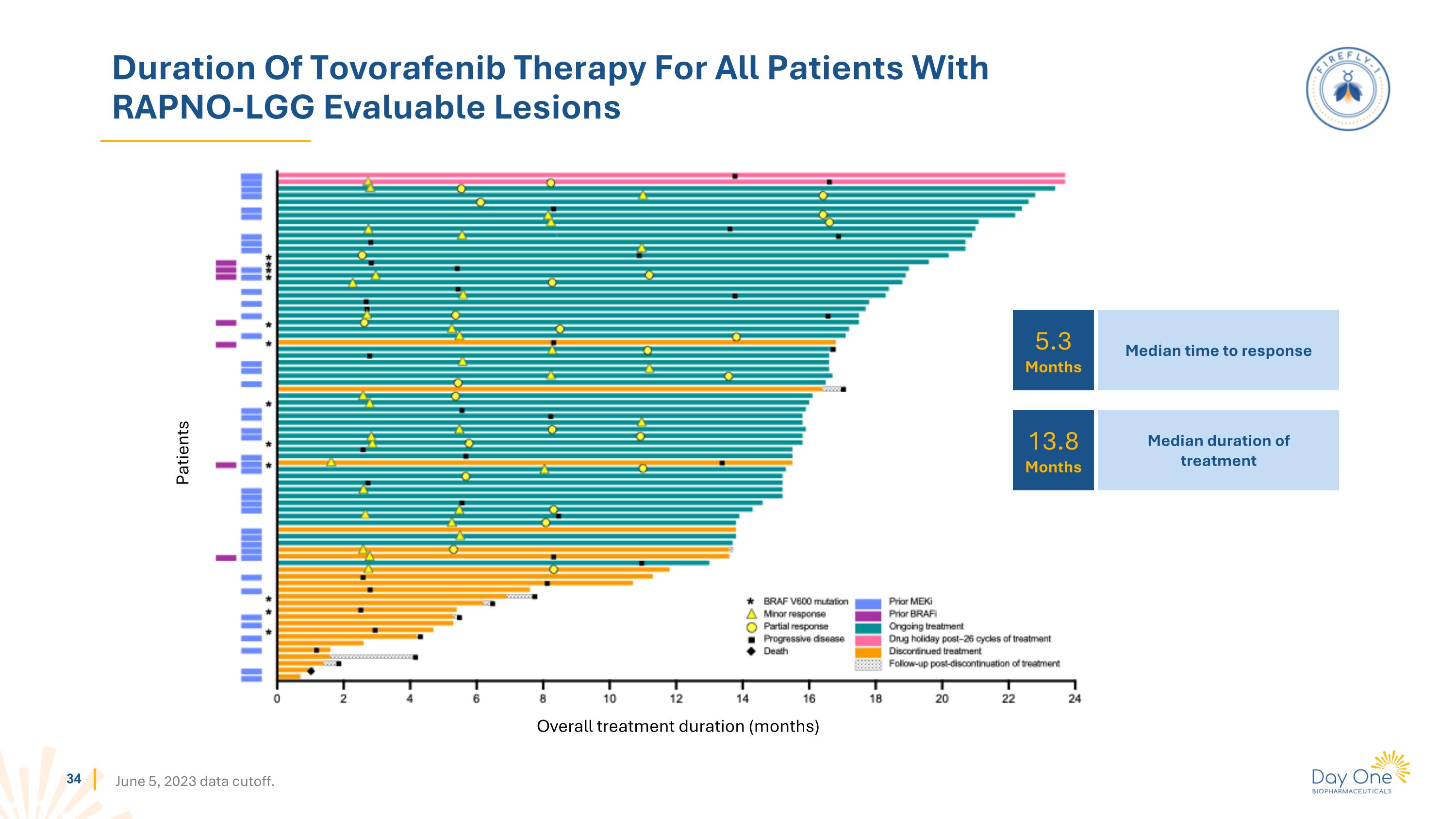
Duration Of Tovorafenib Therapy For All Patients With RAPNO-LGG Evaluable Lesions June 5, 2023 data cutoff. Patients Overall treatment duration (months) 5.3 Months 13.8 Months Median time to response Median duration of treatment
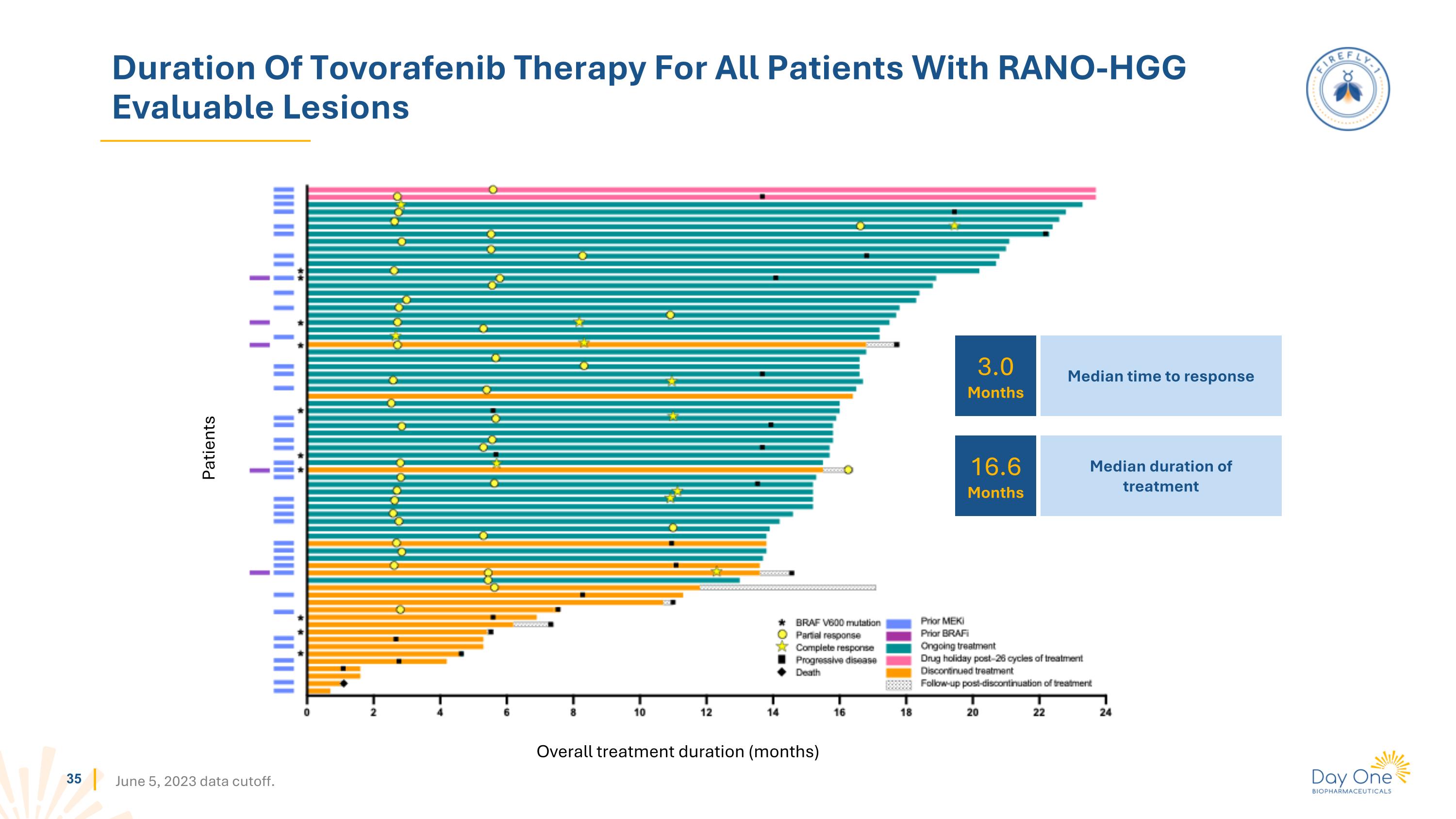
Duration Of Tovorafenib Therapy For All Patients With RANO-HGG Evaluable Lesions Patients Overall treatment duration (months) June 5, 2023 data cutoff. 3.0 Months 16.6 Months Median time to response Median duration of treatment
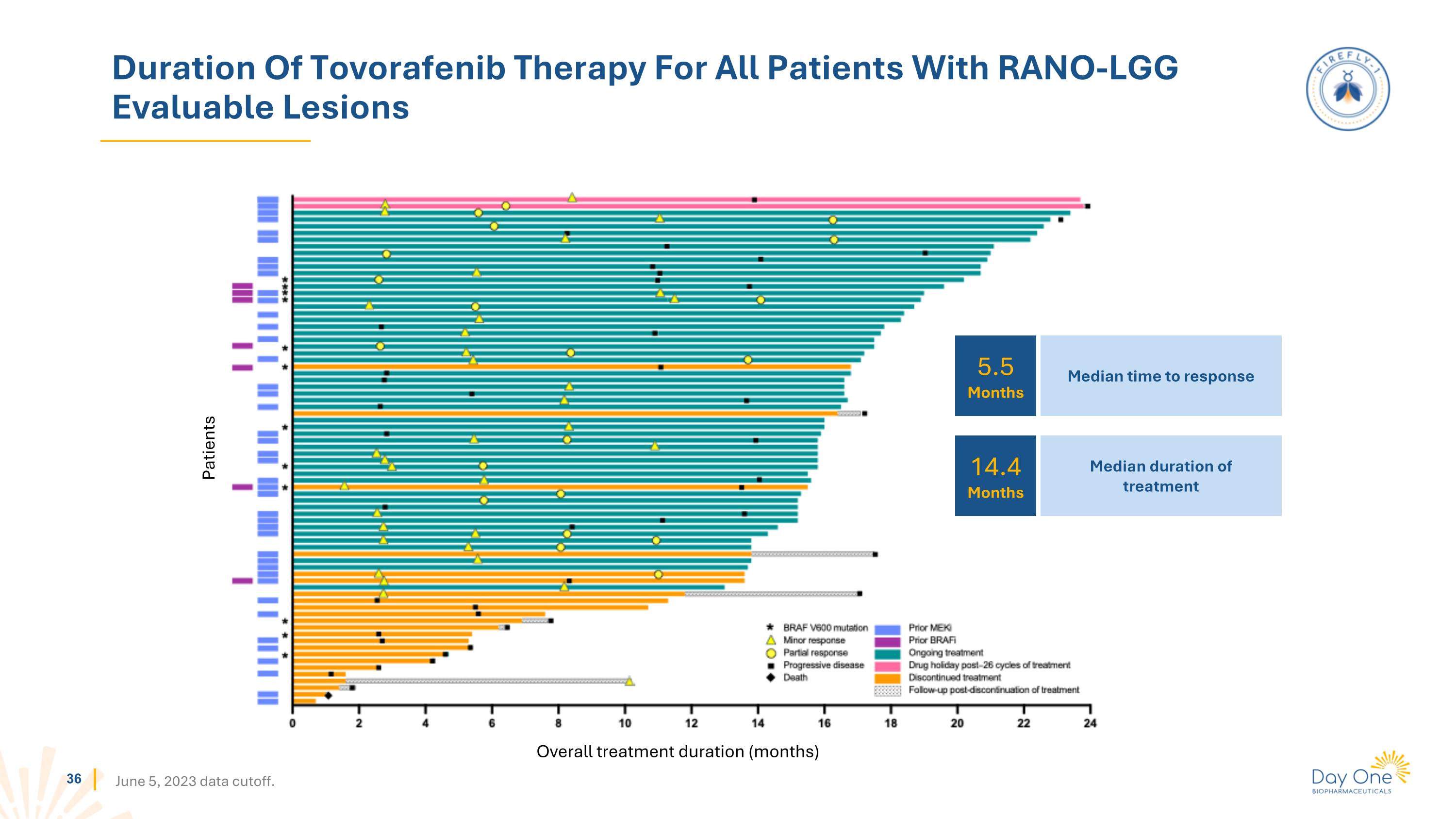
Duration Of Tovorafenib Therapy For All Patients With RANO-LGG Evaluable Lesions Patients Overall treatment duration (months) June 5, 2023 data cutoff. 5.5 Months 14.4 Months Median time to response Median duration of treatment
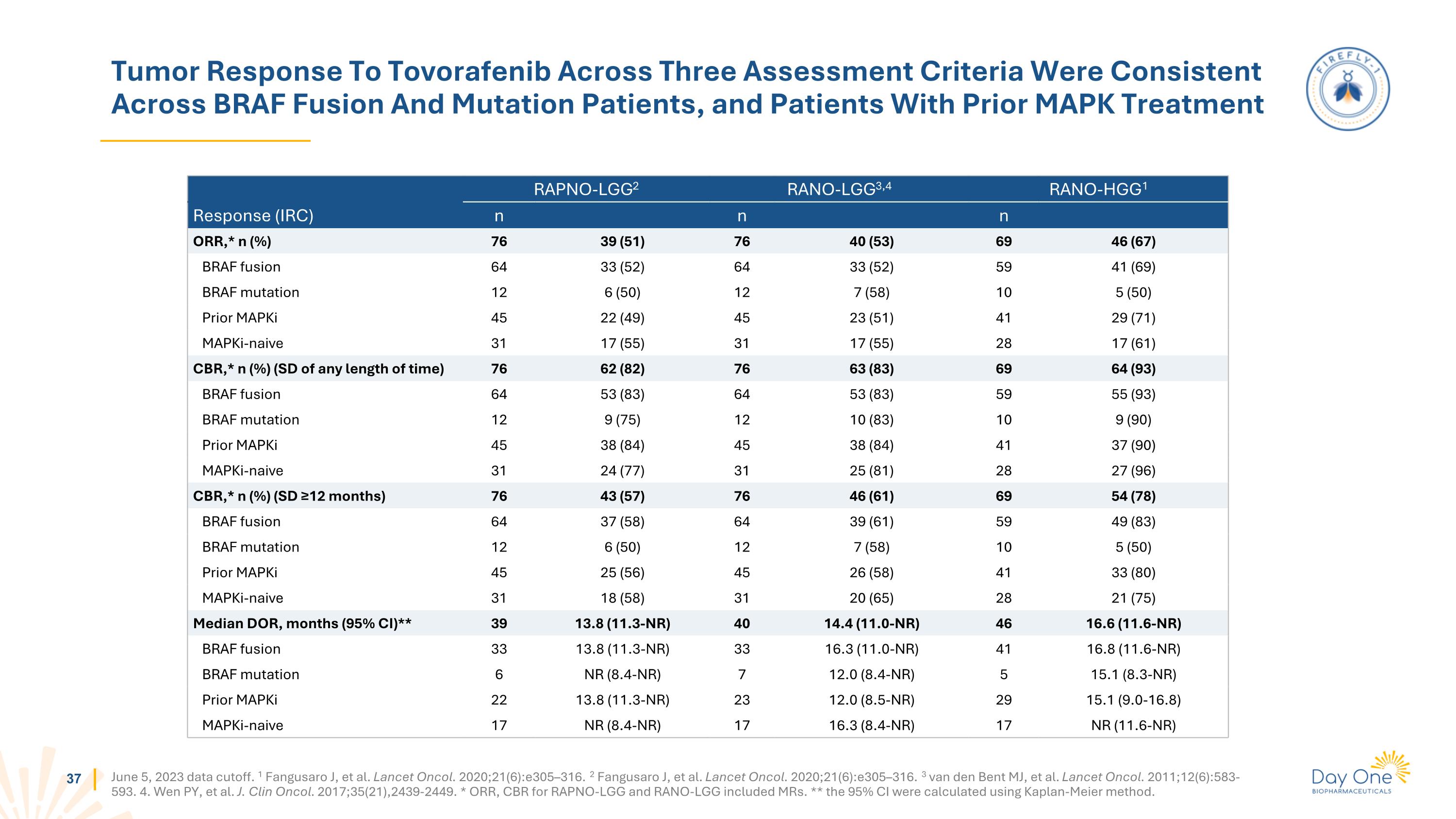
Tumor Response To Tovorafenib Across Three Assessment Criteria Were Consistent Across BRAF Fusion And Mutation Patients, and Patients With Prior MAPK Treatment June 5, 2023 data cutoff. 1 Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. 2 Fangusaro J, et al. Lancet Oncol. 2020;21(6):e305–316. 3 van den Bent MJ, et al. Lancet Oncol. 2011;12(6):583-593. 4. Wen PY, et al. J. Clin Oncol. 2017;35(21),2439-2449. * ORR, CBR for RAPNO-LGG and RANO-LGG included MRs. ** the 95% CI were calculated using Kaplan-Meier method. RAPNO-LGG2 RANO-LGG3,4 RANO-HGG1 Response (IRC) n n n ORR,* n (%) 76 39 (51) 76 40 (53) 69 46 (67) BRAF fusion 64 33 (52) 64 33 (52) 59 41 (69) BRAF mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 22 (49) 45 23 (51) 41 29 (71) MAPKi-naive 31 17 (55) 31 17 (55) 28 17 (61) CBR,* n (%) (SD of any length of time) 76 62 (82) 76 63 (83) 69 64 (93) BRAF fusion 64 53 (83) 64 53 (83) 59 55 (93) BRAF mutation 12 9 (75) 12 10 (83) 10 9 (90) Prior MAPKi 45 38 (84) 45 38 (84) 41 37 (90) MAPKi-naive 31 24 (77) 31 25 (81) 28 27 (96) CBR,* n (%) (SD ≥12 months) 76 43 (57) 76 46 (61) 69 54 (78) BRAF fusion 64 37 (58) 64 39 (61) 59 49 (83) BRAF mutation 12 6 (50) 12 7 (58) 10 5 (50) Prior MAPKi 45 25 (56) 45 26 (58) 41 33 (80) MAPKi-naive 31 18 (58) 31 20 (65) 28 21 (75) Median DOR, months (95% CI)** 39 13.8 (11.3-NR) 40 14.4 (11.0-NR) 46 16.6 (11.6-NR) BRAF fusion 33 13.8 (11.3-NR) 33 16.3 (11.0-NR) 41 16.8 (11.6-NR) BRAF mutation 6 NR (8.4-NR) 7 12.0 (8.4-NR) 5 15.1 (8.3-NR) Prior MAPKi 22 13.8 (11.3-NR) 23 12.0 (8.5-NR) 29 15.1 (9.0-16.8) MAPKi-naive 17 NR (8.4-NR) 17 16.3 (8.4-NR) 17 NR (11.6-NR)
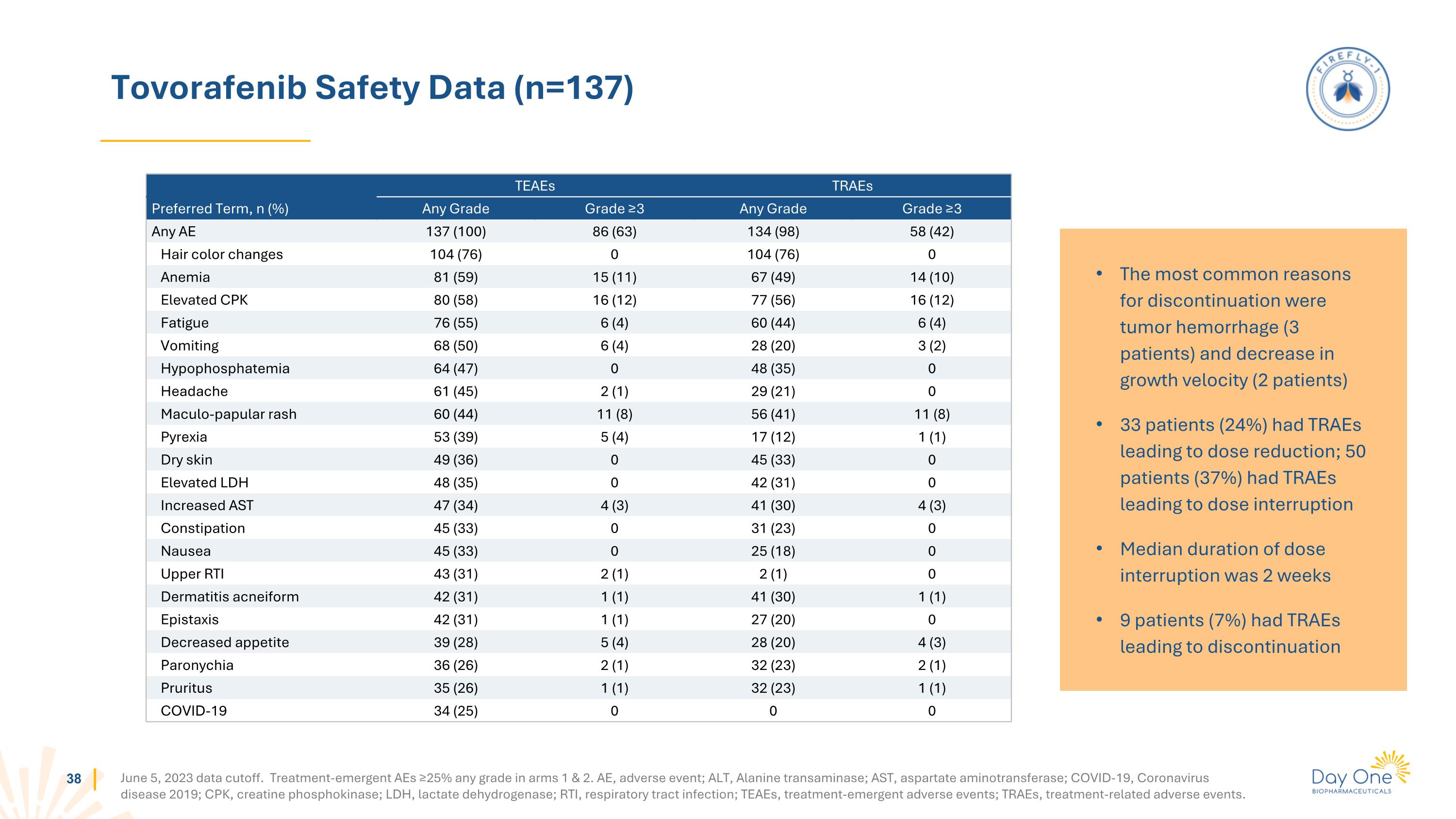
Tovorafenib Safety Data (n=137) June 5, 2023 data cutoff. Treatment-emergent AEs ≥25% any grade in arms 1 & 2. AE, adverse event; ALT, Alanine transaminase; AST, aspartate aminotransferase; COVID-19, Coronavirus disease 2019; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; RTI, respiratory tract infection; TEAEs, treatment-emergent adverse events; TRAEs, treatment-related adverse events. TEAEs TRAEs Preferred Term, n (%) Any Grade Grade ≥3 Any Grade Grade ≥3 Any AE 137 (100) 86 (63) 134 (98) 58 (42) Hair color changes 104 (76) 0 104 (76) 0 Anemia 81 (59) 15 (11) 67 (49) 14 (10) Elevated CPK 80 (58) 16 (12) 77 (56) 16 (12) Fatigue 76 (55) 6 (4) 60 (44) 6 (4) Vomiting 68 (50) 6 (4) 28 (20) 3 (2) Hypophosphatemia 64 (47) 0 48 (35) 0 Headache 61 (45) 2 (1) 29 (21) 0 Maculo-papular rash 60 (44) 11 (8) 56 (41) 11 (8) Pyrexia 53 (39) 5 (4) 17 (12) 1 (1) Dry skin 49 (36) 0 45 (33) 0 Elevated LDH 48 (35) 0 42 (31) 0 Increased AST 47 (34) 4 (3) 41 (30) 4 (3) Constipation 45 (33) 0 31 (23) 0 Nausea 45 (33) 0 25 (18) 0 Upper RTI 43 (31) 2 (1) 2 (1) 0 Dermatitis acneiform 42 (31) 1 (1) 41 (30) 1 (1) Epistaxis 42 (31) 1 (1) 27 (20) 0 Decreased appetite 39 (28) 5 (4) 28 (20) 4 (3) Paronychia 36 (26) 2 (1) 32 (23) 2 (1) Pruritus 35 (26) 1 (1) 32 (23) 1 (1) COVID-19 34 (25) 0 0 0 The most common reasons for discontinuation were tumor hemorrhage (3 patients) and decrease in growth velocity (2 patients) 33 patients (24%) had TRAEs leading to dose reduction; 50 patients (37%) had TRAEs leading to dose interruption Median duration of dose interruption was 2 weeks 9 patients (7%) had TRAEs leading to discontinuation