美國
證券交易委員會
華盛頓特區20549
表格
(標記一個)
| | 根據1934年證券交易法第13或15(d)條款的季度報告。 |
截至2024年6月30日季度結束
或
| | 根據1934年證券交易法第13或15(d)條款的過渡報告 |
過渡期從________到________
委員會檔案編號:
柯丹恩生技股份有限公司。
(依憑章程所載的完整登記名稱)
| | |
| (依據所在地或其他管轄區) 的註冊地或組織地點) | (國稅局雇主識別號碼) |
|
| |
| (總部辦公地址) | (郵政編碼) |
(
MNPR’(納斯達克資本市場)
10578 Science Center Drive, Suite 200
加州聖地牙哥 92121
(如與上次報告不同,列明前名稱、前地址及前財政年度)
根據法案第12(b)條規定註冊的證券:
| 每種類別的名稱 | 交易 標的 | 每個註冊交易所的名稱 | ||
| | | 輝瑞公司面臨數起分開的訴訟,這些訴訟仍在進行中,需等待第三項索賠條款的裁決。2023年9月,我們與輝瑞公司同意合併2022和2023年的訴訟,並將審判日期從2024年11月推遲至2025年上半年,具體時間將由法院確定。 (納斯達克全球精選市場) |
請勾選以下項目,以判定在過去12個月(或更短期間,該註冊人被要求提交報告)內所有根據1934年證券交易法第13條或第15(d)條要求提供報告的報告是否已經提交,並且該註冊人在過去90天中是否受到提交報告的要求。
在前12個月內(或公司需要提交這些文件的較短時間內),公司是否已通過選中標記表明已閱讀並提交了應根據S-t法規第405條規定(本章第232.405條)提交的所有互動式數據文件?
請勾選指示登記者是否為大型快速提交人、快速提交人、非快速提交人、較小的報告公司或新興成長型公司。請參閱交易所法規120億2條,了解「大型快速提交人」、「快速提交人」、「較小的報告公司」和「新興成長型公司」的定義。
| 大型加速歸檔人 | ☐ | 加速歸檔人 | ☐ |
| | ☒ | 小型報告公司 | |
| 新興成長型企業 | |
如果一家新興成長型公司,請用勾選標記表示該申報人已選擇不使用根據證交所法案13(a)條款提供的任何新的或修訂過的財務會計準則的延長過渡期。
在核准的名冊是否屬於殼公司(如股市法規第1202條所定義之意義)方面,請用勾選符號表示。是
截至2024年11月1日,登記人持有25,777,793股已發行股份中,有
有關前瞻性陳述的特別提示
本季度10-Q表格中包含有關我們業務、運營和財務表現與狀況的前瞻性陳述,以及我們對業務、運營和財務表現與狀況的計劃、目標和期望。本文件中包含的非歷史事實陳述可能被視為前瞻性陳述。這些陳述涉及已知和未知風險、不確定因素和其他重要因素,有時超出我們的控制範圍,可能導致我們的實際結果、表現或成就與前瞻性陳述所暗示的任何未來結果、表現或成就有實質不同。
「預期」、「相信」、「考慮」、「持續」、「可能」、「估計」、「期望」、「打算」、「可以」、「可能」、「計劃」、「潛力」、「預測」、「項目」、「應該」、「目標」、「將」或「將」或這些詞的否定形式或其他類似表達的用語,意在識別前瞻性陳述。本報告中包含的前瞻性陳述包括但不限於以下方面:
• 我們臨床試驗展示我們藥物候選者安全性和有效性的可能性;
• 我們當前臨床試驗的時間和進展,這些臨床試驗的預期結果以及我們未來臨床試驗啟動的時間;
• 我們與目前和未來藥物候選者的臨床發展相關的計劃,包括要評估的大小,數量和疾病領域;
• 強生("J&J")與PIPE-307臨床發展相關的計劃;
• 我們的臨床轉化方法,以及我們識別和開發能夠通過針對與特定臨床受損相關的生物途徑來改變疾病進程的藥物候選者的能力,進而可能治療神經科學,炎症和免疫學("NI&I")疾病;
• 我們藥物候選者市場機遇的規模;
• 我們藥物候選者市場接受度和臨床效益的速度和程度;
• 有關我們的藥物候選品取得商業化,如果獲批准的計劃;
• 競爭療法和可能可用的技術的成功;
• 我們的藥物候選品的有益特性、安全性、效力、治療效果和潛在優勢;
• 我們的藥物候選品的監管申報和批准的時間或可能性;
• 我們獲得和保持對我們的藥物候選品的監管批准以及我們的藥物候選品符合現有或未來監管標準的能力;
• 我們關於進一步開發和製造我們的藥物候選品的計劃,包括我們可能探索的附加適應症;
• 我們成功識別並完成許可進入或其他取得其他藥物候選品、技術、產品或業務的交易的能力;
• 我們吸引並與具有開發、監管、製造和商業化專業知識的第三方進行商業安排的能力;
• 我們的計劃和能力,包括獲取或保護知識產權,包括現有專利期限的延長(如有),以及我們獲得和保留知識產權監管權和監管保護的能力;
• 我們保留高級管理階層的能力;
• 需要招聘額外人員以及我們吸引和保留這些人員的能力;
• 關於我們營運週期、支出、資本需求和獲得額外融資的估計準確性;
• 我們現有資本資源是否足夠支持未來營業費用和資本支出需求;
• 我們預期將符合2012年初創企業促進法案的新興成長企業或較小的報告公司資格的時間段;
• 我們預期使用我們現有的現金、現金等及市場有價證券;
• 其他風險和不確定因素,包括本季度報告的第二部分第1A項“風險因素”中描述的內容。
本季度報告中的任何前瞻性陳述均反映了我們對未來事件或未來財務表現的當前看法,涉及已知和未知的風險、不確定因素和其他可能導致我們實際結果、表現或成就與這些前瞻性陳述所隱含或明示的任何未來結果、表現或成就有實質不同的因素。可能導致實際結果與當前預期有實質不同的因素包括,但不限於,本季度報告中列出的其他事項,該列出事項請參閱第II部分第1A項“風險因素”。鑑於這些不確定性,您不應過度依賴這些前瞻性陳述,也不應將前瞻性陳述視為未來事件的預測。除非法律要求,我們不承諾出於任何原因更新或修訂這些前瞻性陳述,即使將來有新信息可得
除非上下文另有指示,本季度報告中對“Contineum”,“公司”,“我們”,“我們的”和“我們”一詞的引用均指Contineum Therapeutics, Inc.,對我們的“普通股”一詞的引用則指我們的投票類A普通股。
目 錄
| 頁面 |
||
| 第一部分。 |
||
| 项目1。 |
||
| 项目2。 |
||
| 项目3。 |
||
| 項目 4。 |
||
| 第二部分。 |
||
| 项目1。 |
||
| 项目1A。 |
||
| 项目2。 |
||
| 项目3。 |
||
| 項目 4。 |
||
| 项目5。 |
||
| 第6項。 |
||
簡明資產負債表
(未經審計)
(以千為單位,股數和面值數據除外)
| 2024年9月30日 | 2023年12月31日 | |||||||
| 資產 | ||||||||
| 流動資產: | ||||||||
| 現金及現金等價物 | $ | $ | ||||||
| 有價證券 | ||||||||
| 預付費用及其他流動資產 | ||||||||
| 全部流動資產 | ||||||||
| 物業及設備,扣除折舊後淨值 | ||||||||
| 其他長期資產 | ||||||||
| 營運租賃權使用資產 | ||||||||
| 資產總額 | $ | $ | ||||||
| 負債、可轉換優先股和股東權益(赤字) | ||||||||
| 流動負債: | ||||||||
| 應付賬款 | $ | $ | ||||||
| 應計費用 | ||||||||
| 營運租賃負債的流動部分 | ||||||||
| 流動負債合計 | ||||||||
| 其他長期負債 | ||||||||
| 扣除當期償還後之經營租賃負債淨額 | ||||||||
| 總負債 | ||||||||
| 2024年6月30日和2023年12月31日分別發行和流通的可轉換優先股,分別為$125,292.00、$100,912.65和$93,890.20。清算優先權為$242,381、$187,780、$108,696和$96,106。 面額為0.0001; 2024年9月30日核准、發行或流通的股份為;核准股份— 2023年12月31日;已發行和流通股份— 5,421,872 | ||||||||
| 股東權益(赤字): | ||||||||
| A類普通股,$ 票面價值;核准股份— 和 分別為2024年9月30日和2023年12月31日核准、發行和流通的股份— 和 於2024年9月30日和2023年12月31日,分別為 | ||||||||
| B類普通股,$ 面額;授權股份- 截至2024年9月30日;已發行及流通股票- 截至2024年9月30日; 2023年12月31日時授權、已發行或流通股份 | ||||||||
| 優先股,面額$0.01,授權股數為5,000,000股,發行且流通股數為截至2024年6月30日和2023年12月31日之184,668,188股和181,364,180股。 面值;授權股數─ 截至2024年9月30日; 於2024年9月30日已發行或流通的股份; 於2023年12月31日已授權、發行或流通的股份 | ||||||||
| 資本公積金額外 | ||||||||
| 累積虧損 | ( | ) | ( | ) | ||||
| 累積其他綜合收益 | ||||||||
| 股東權益總額(赤字) | ( | ) | ||||||
| 負債總額、可轉換優先股和股東權益(赤字) | $ | $ | ||||||
所附附註為這些未經審計簡明財務報表的一部分。
綜合損益表摘要
(未經審計)
(以千為單位,除每股股份和每股資料外)
| 截至9月30日的三個月 |
截至9月30日的九個月 |
|||||||||||||||
| 2024 |
2023 |
2024 |
2023 |
|||||||||||||
| 營業收入: |
||||||||||||||||
| 許可營業收入 |
$ | $ | $ | $ | ||||||||||||
| 營業費用: |
||||||||||||||||
| 研發費用 |
||||||||||||||||
| 總務與行政 |
||||||||||||||||
| 營業費用總計 |
||||||||||||||||
| 營業利益(損失) |
( |
) | ( |
) | ( |
) | ||||||||||
| 其他收入(費用): |
||||||||||||||||
| 利息收入 |
||||||||||||||||
| 利息費用 |
( |
) | ||||||||||||||
| 公允價值調整權證負債 |
( |
) | ||||||||||||||
| 投資者權利和義務負債公平值變動 |
||||||||||||||||
| 其他費用,淨額 |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| 其他收益合計 |
||||||||||||||||
| 稅前收入(虧損) |
( |
) | ( |
) | ( |
) | ||||||||||
| 所得稅賦(減)益 |
( |
) | ||||||||||||||
| 凈利潤(損失) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 其他綜合損益: |
||||||||||||||||
| 可銷售證券未實現收益(損失) |
( |
) | ( |
) | ||||||||||||
| 綜合收益(損失) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 歸屬於普通股股東的凈利潤(損失),基本 |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 歸屬於普通股股東的凈利潤(損失),稀釋 |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 基本每股盈利(損失) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 稀釋每股盈利(損失) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| 基本流通股平均數 |
||||||||||||||||
| 稀釋流通股平均數 |
||||||||||||||||
_____________
| (a) | 基本和稀釋後的每股金額對A類和B類股票是相同的。 |
附帶說明是這些未經審計的簡明基本報表的重要組成部分。
CONDENSED STATEMENTS OF CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT)
(unaudited)
(以千爲單位,股份數據除外)
| A班和B班 | 額外的 | 累計其他 | 股東總數 | |||||||||||||||||||||||||||||
| 可轉換優先股 | 普通股 | 實收股本 | 綜合 | 累計 | 股權 | |||||||||||||||||||||||||||
| 股份 | 金額 | 股份 | 金額 | 資本 | 收入(損失) | 虧損 | (赤字) | |||||||||||||||||||||||||
| 2023年12月31日餘額 | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 淨虧損 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 可供出售證券未實現減值損失 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 2024年3月31日結存餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| 在首次公開招股中發行普通股,扣除發行成本$ | ||||||||||||||||||||||||||||||||
| 首次公開發行時可轉換優先股轉爲普通股 | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 將權證從負債重新分類爲權益 | — | — | ||||||||||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 淨虧損 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 可供出售證券未實現減值損失 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 2024年6月30日餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 淨虧損 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 可交易證券的未實現收益 | — | — | ||||||||||||||||||||||||||||||
| 2024年9月30日的結餘 | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||||||||||
| 額外的 | 累積其他 | 總計 | ||||||||||||||||||||||||||||||
| 可轉換優先股 | A類普通股 | 實收股本 | 綜合 | 累計 | 股東的 | |||||||||||||||||||||||||||
| 股份 | 金額 | 股份 | 金額 | 資本 | 虧損 | 虧損 | 虧損 | |||||||||||||||||||||||||
| 2022年12月31日的餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| 有關可回購的普通股的股份歸屬 | — | — | — | — | — | |||||||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 淨虧損 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 可交易證券的未實現收益 | — | — | ||||||||||||||||||||||||||||||
| 2023年3月31日的餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| 受回購限制的普通股歸屬 | — | — | — | — | — | |||||||||||||||||||||||||||
| 發行C系列可轉換優先股,扣除103美元的發行成本 | ||||||||||||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 回購期權 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 淨利潤 | — | — | ||||||||||||||||||||||||||||||
| 可供出售證券未實現減值損失 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 2023年6月30日的餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
| 股份歸屬的普通股可回購 | — | — | — | — | — | |||||||||||||||||||||||||||
| 發行C輪可轉換優先股,扣除發行成本$ | ||||||||||||||||||||||||||||||||
| 行使股票期權 | ||||||||||||||||||||||||||||||||
| 基於股票的補償 | — | — | — | |||||||||||||||||||||||||||||
| 淨利潤 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 可供出售證券未實現減值損失 | — | — | ( | ) | ( | ) | ||||||||||||||||||||||||||
| 2023年9月30日的餘額 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||
附帶說明是這些未經審計的簡明基本報表的重要組成部分。
現金流量表
(未經審計)
(以千爲單位)
| 截至9月30日的九個月 |
||||||||
| 2024 |
2023 |
|||||||
| 經營活動 |
||||||||
| 淨利潤(損失) |
$ | ( |
) | $ | ||||
| 調整淨利潤(損失)以達到經營活動產生的現金流量需求: |
||||||||
| 折舊和攤銷 |
||||||||
| 非現金租賃費用 |
||||||||
| 基於股票的補償 |
||||||||
| 債務折讓和債務發行成本的增加 |
( |
) | ||||||
| 投資中權益/貼現淨溢價/折價增加 |
( |
) | ( |
) | ||||
| 權證責任公允價值變動 |
( |
) | ||||||
| 設備銷售所得的收益 |
( |
) | ||||||
| (收益)虧損在可交易證券 |
( |
) | ||||||
| 投資者權益和義務負債公允價值變動 |
( |
) | ||||||
| 營運資產和負債的變化 |
||||||||
| 預付費用及其他流動資產 |
( |
) | ||||||
| 其他長期資產 |
( |
) | ||||||
| 應付賬款 |
||||||||
| 應計費用 |
( |
) | ||||||
| 營運租賃負債 |
( |
) | ||||||
| 經營活動產生的淨現金流量 |
( |
) | ||||||
| 投資活動 |
||||||||
| 購置固定資產等資產支出 |
( |
) | ( |
) | ||||
| 出售設備的收益 |
||||||||
| 購買有市場流通的證券 |
( |
) | ( |
) | ||||
| 市場證券的銷售和到期 |
||||||||
| 投資活動中使用的淨現金 |
( |
) | ( |
) | ||||
| 籌資活動 |
||||||||
| 首次公開發行普通股後的募集收入,扣除承銷折扣、佣金和其他發行成本 |
||||||||
| 發行C輪可轉換優先股所得款項,扣除發行費用 |
||||||||
| 債務本金償還 |
( |
) | ||||||
| 行使股票期權所得 |
||||||||
| 融資活動提供的淨現金 |
||||||||
| 現金及現金等價物淨增加額 |
||||||||
| 期初現金及現金等價物餘額 |
||||||||
| 期末現金及現金等價物 |
$ | $ | ||||||
| 非現金投資和融資活動補充披露 |
||||||||
| 首次公開發行時可轉換優先股轉爲普通股 |
$ | $ | ||||||
| 將權證從負債重新分類爲權益 |
$ | $ | ||||||
| 將去年支付的遞延發行成本重新分類爲股本 |
$ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
1. Organization and Basis of Presentation
Organization and Nature of Operations
Contineum Therapeutics, Inc. (the “Company”), is a clinical stage biopharmaceutical company focused on discovering and developing novel, oral small molecule therapies for neuroscience, inflammation and immunology indications with high unmet need. The Company, formerly named Sirocco Therapeutics, Inc. (“Sirocco” or “legacy Sirocco”), Inception 3, Inc. (“Inception”) and Versense Pharmaceuticals, Inc. (“Versense”), was incorporated in the state of Delaware in 2009 as Versense. Versense changed its name to Inception on October 25, 2011, and commenced active operations on July 13, 2012. In May 2018, Inception changed its name to Sirocco. A separate entity named Pipeline Therapeutics, Inc. (“legacy Pipeline”) was founded and incorporated in the state of Delaware on May 9, 2017. On May 7, 2019, legacy Sirocco acquired legacy Pipeline in a merger transaction. As of December 31, 2019, legacy Pipeline was a wholly owned subsidiary of legacy Sirocco. In January 2020, legacy Pipeline was merged into legacy Sirocco and ceased to exist, and legacy Sirocco changed its name to Pipeline Therapeutics, Inc. In November 2023, Pipeline Therapeutics, Inc. changed its name to Contineum Therapeutics, Inc.
Reverse Stock Split
On April 1, 2024, the Company filed an amendment to its fourth amended and restated certificate of incorporation as amended and effected a 1-for-
Initial Public Offering
On April 9, 2024, the Company closed its initial public offering (“IPO”), pursuant to which it issued and sold an aggregate of
In connection with the closing of its IPO, on April 9, 2024, the Company’s certificate of incorporation was amended and restated to (i) authorize
Liquidity and Capital Resources
Since its inception, the Company has devoted substantially all its resources to research and development activities, business planning, establishing and maintaining its intellectual property portfolio, hiring personnel, raising capital to support and expand such activities and providing general and administrative support for these operations. The Company incurred a net loss of $
As of September 30, 2024, the Company had cash, cash equivalents and marketable securities of $
As the Company continues to pursue its business plan, it expects to finance its operations through both public and private sales of equity, debt financings or other commercial arrangements, which could include income from collaborations, strategic partnerships or marketing, distribution, licensing or other strategic arrangements with third parties. However, there can be no assurance that any additional financing or strategic transactions will be available to the Company on acceptable terms, if at all. If events or circumstances occur such that the Company does not obtain additional funding, it may need to delay, reduce or eliminate its product development or future commercialization efforts, which could have a material adverse effect on the Company’s business, results of operations, financial condition and cash flows. Further, if the Company raises funds through licensing or other similar arrangements with third parties, it may be required to relinquish valuable rights to its technology, future revenue streams, research programs or drug candidates or may be required to grant licenses on terms that may not be favorable to it and/or may reduce the value of its common stock.
Unaudited Interim Condensed Financial Statements
The condensed balance sheet as of September 30, 2024, condensed statements of operations and comprehensive income (loss) and condensed statements of convertible preferred stock and stockholders’ equity (deficit) for the three and nine months ended September 30, 2024 and 2023, and condensed statements of cash flows for the nine months ended September 30, 2024 and 2023, and related notes to condensed financial statements are unaudited. These unaudited interim condensed financial statements have been prepared on the same basis as the Company’s annual financial statements and, in the opinion of management, reflect all adjustments (consisting only of normal recurring adjustments) that are necessary for the fair statement of the Company’s financial position, results of operations and cash flows for the periods presented. The condensed results of operations for the three and nine months ended September 30, 2024 are not necessarily indicative of the results to be expected for the full year or for any other future annual or interim period. The condensed balance sheet as of December 31, 2023 included herein was derived from the audited financial statements as of that date. These interim unaudited condensed financial statements should be read in conjunction with the Company’s audited financial statements for the year ended December 31, 2023 included in the Company’s Form S-1, as amended (File No. 333-278003) as filed with the Securities and Exchange Commission ("SEC") pursuant to Rule 424(b) of the Securities Act of 1933, as amended, on April 1, 2024 and declared effective by the SEC on April 4, 2024 (the "Registration Statement").
2. Summary of Significant Accounting Policies
During the nine months ended September 30, 2024, there were no significant changes to the Company's significant accounting policies as described in the Company's Registration Statement.
Basis of presentation
The Company has prepared the accompanying condensed financial statements in accordance with U.S. generally accepted accounting principles (“GAAP”) and the requirements of the SEC for interim reporting. As permitted under those rules, certain footnotes or other financial information that are normally required by GAAP can be condensed or omitted. The financial statements are presented in U.S. dollars. Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification ("ASC") and Accounting Standards Updates ("ASU") promulgated by the Financial Accounting Standards Board ("FASB").
Use of estimates
During the nine months ended September 30, 2024, there were no significant changes to the Company's accounting estimates as described in the Company's Registration Statement.
Recently Issued Accounting Pronouncements
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures ("ASU 2023-07"). This standard requires a public entity to disclose significant segment expenses and other segment items on an interim and annual basis. Additionally, it requires a public entity to disclose the title and position of the Chief Operating Decision Maker. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. A public entity should apply the amendments in this ASU retrospectively to all prior periods presented in the financial statements. The Company is currently evaluating the impact of this guidance on its financial statements.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures ("ASU 2023-09"). This standard requires disaggregated information about a reporting entity’s effective tax rate reconciliation as well as information on income taxes paid. ASU 2023-09 is effective for public entities with annual periods beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of this guidance on its financial statements.
3. Marketable Securities
The Company invests its excess cash in marketable securities, including debt securities, commercial paper, asset-backed securities, Yankee debt, certificate of deposit, and U.S. Government agency securities.
The following table summarizes the amortized cost and fair value of the Company’s marketable securities by major investment category (in thousands):
| As of September 30, 2024 |
||||||||||||||||||
| Unrealized |
||||||||||||||||||
| Maturity in Years | Amortized Cost |
Gains |
Losses |
Fair Value |
||||||||||||||
| U.S. Government agency securities |
3 years or less | $ | $ | $ | ( |
) | $ | |||||||||||
| Certificate of deposit |
Less than 1 | |||||||||||||||||
| Corporate debt securities |
3 years or less | ( |
) | |||||||||||||||
| Commercial paper |
Less than 1 | ( |
) | |||||||||||||||
| Yankee debt |
Less than 1 | |||||||||||||||||
| Asset-backed securities |
2 years or less | |||||||||||||||||
| $ | $ | $ | ( |
) | $ | |||||||||||||
| As of December 31, 2023 |
||||||||||||||||||
| Unrealized |
||||||||||||||||||
| Maturity in Years | Amortized Cost |
Gains |
Losses |
Fair Value |
||||||||||||||
| U.S. Government agency securities |
2 years or less | $ | $ | $ | $ | |||||||||||||
| Certificate of deposit |
Less than 1 | |||||||||||||||||
| Corporate debt securities |
2 years or less | ( |
) | |||||||||||||||
| Commercial paper |
Less than 1 | ( |
) | |||||||||||||||
| Yankee debt |
Less than 1 | |||||||||||||||||
| Asset-backed securities |
3 years or less | ( |
) | |||||||||||||||
| $ | $ | $ | ( |
) | $ | |||||||||||||
The Company regularly reviews the securities in an unrealized loss position and evaluates the current expected credit loss by considering factors such as historical experience, market data, issuer-specific factors, current and expected future economic conditions. The Company has no requirement or intention to sell these securities before maturity or recovery of their amortized cost basis. As of September 30, 2024, the Company did record an allowance for credit loss related to its investment portfolio.
4. Fair Value Measurements
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1—Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities.
Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability.
Level 3—Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (i.e. supported by little or no market activity).
Assets and liabilities measured at fair value on a recurring basis are as follows (in thousands):
| Fair Value Measurements Using |
||||||||||||||||
| Quoted Prices in |
Significant |
|||||||||||||||
| Active Markets |
Significant Other |
Unobservable |
||||||||||||||
| for Identical |
Observable |
Inputs |
||||||||||||||
| Total |
Assets (Level 1) |
Inputs (Level 2) |
(Level 3) |
|||||||||||||
| As of September 30, 2024: |
||||||||||||||||
| Assets: |
||||||||||||||||
| Cash equivalents |
$ | $ | $ | $ | ||||||||||||
| U.S. Government agency securities |
||||||||||||||||
| Certificates of deposits |
||||||||||||||||
| Corporate debt securities |
||||||||||||||||
| Commercial paper |
||||||||||||||||
| Yankee debt |
||||||||||||||||
| Asset-backed securities |
||||||||||||||||
| Total financial assets |
$ | $ | $ | $ | ||||||||||||
| As of December 31, 2023: |
||||||||||||||||
| Assets: | ||||||||||||||||
| Cash equivalents |
$ | $ | $ | $ | ||||||||||||
| U.S. Government agency securities |
||||||||||||||||
| Certificates of deposits |
||||||||||||||||
| Corporate debt securities |
||||||||||||||||
| Commercial paper |
||||||||||||||||
| Yankee debt |
||||||||||||||||
| Asset-backed securities |
||||||||||||||||
| Total financial assets |
$ | $ | $ | $ | ||||||||||||
| Liabilities: |
||||||||||||||||
| Preferred stock warrant liability |
( |
) | ( |
) | ||||||||||||
| Total financial liabilities |
$ | ( |
) | $ | $ | $ | ( |
) | ||||||||
The carrying amounts of the Company’s financial instruments, including cash, cash equivalents and marketable securities, prepaid and other current assets, accounts payable, and accrued liabilities, approximate fair value due to their short maturities. Included in cash and cash equivalents at September 30, 2024 and December 31, 2023 are money market funds with a carrying value and fair value of $
Warrant
Upon the closing of the IPO, the warrant to purchase shares of Series B preferred stock converted to a warrant to purchase shares of Class A common stock, and upon conversion the warrant met the equity classification requirements and was reclassified to equity. The warrant was remeasured to fair value immediately prior to the conversion to a common stock warrant and the Company recognized changes in the fair value of the warrant liability until April 9, 2024.
As of December 31, 2023, the Company had a preferred stock warrant liability (included on the balance sheet under other long-term liabilities), which consisted of the fair value of a warrant to purchase Series B convertible preferred stock and was based on significant unobservable inputs, which represent a Level 3 measurement within the fair value hierarchy. The Company classified this warrant as a liability on its balance sheets and remeasured to fair value at each reporting date, and the Company recognized changes in the fair value of the warrant liability as a component of other income (expense) in its condensed statements of operations and comprehensive income (loss).
The Company’s valuation of the preferred stock warrant as of April 9, 2024 and December 31, 2023 utilized the Black-Scholes option-pricing model. The quantitative elements associated with the Company’s Level 3 inputs impacting the fair value measurement of the preferred stock warrant liability include the fair value per share of the underlying Series B convertible preferred stock, the remaining contractual term of the warrant, risk-free interest rate, expected dividend yield and expected volatility of the price of the underlying preferred stock. The most significant assumption in the Black-Scholes option-pricing model impacting the fair value of the preferred stock warrant is the fair value of the Company’s Series B convertible preferred stock as of each remeasurement date. The Company determines the fair value per share of the underlying preferred stock by taking into consideration its most recent sales of its convertible preferred stock as well as additional factors that the Company deems relevant. The Company historically has been a private company and lacks company-specific historical and implied volatility information of its stock. Therefore, it estimates its expected stock volatility based on the historical volatility of publicly traded peer companies for a term equal to the remaining contractual term of the warrant.
The risk-free interest rate is determined by reference to the U.S. Treasury yield curve for time periods approximately equal to the remaining contractual term of the warrant. The Company has estimated a
Significant increases or decreases in any of these inputs in isolation would result in a significantly different fair value measurement. An increase in the risk-free interest rate, and/or an increase in the remaining contractual term or expected volatility, and/or an increase in the fair value of the convertible preferred stock would result in an increase in the fair value of the warrant.
The following table summarizes the change in fair value of the preferred stock warrant liability, a Level 3 recurring fair value measurement, for the nine months ended September 30, 2024 (in thousands):
| Warrant Liability |
||||
| Balance at December 31, 2023 |
$ | |||
| Change in fair value of warrant liability |
||||
| Balance at April 9, 2024 |
$ | |||
| Reclassification to equity |
( |
) | ||
| Balance at September 30, 2024 |
$ | |||
5. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| September 30, |
December 31, |
|||||||
| 2024 |
2023 |
|||||||
| Accrued compensation expenses |
$ | $ | ||||||
| Accrued research and development expenses |
||||||||
| Accrued professional and consulting expenses |
||||||||
| Other accrued expenses |
||||||||
| Total accrued expenses |
$ | $ | ||||||
6. Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Upon the closing of the IPO, the Company’s outstanding convertible preferred stock automatically converted into
Convertible Preferred Stock
As of December 31, 2023, the Company’s Series A, Series A-1, Series B, and Series C convertible preferred stock were classified as temporary equity in the accompanying condensed balance sheet given that a majority of the Company’s board of director seats were held and/or voted upon by convertible preferred stockholders, and those convertible preferred stockholders could cause certain events to occur requiring redemption of the preferred stock that were outside of the Company’s control. The Company did not adjust the carrying values of the convertible preferred stock to the respective liquidation preferences of such shares as the instruments were not currently redeemable and it was not probable that the instruments would become redeemable.
The authorized, issued, and outstanding shares of convertible preferred stock as of December 31, 2023 consisted of the following:
| Shares Authorized | Shares Issued and Outstanding | Liquidation Preference (in thousands) | ||||||||||
| Series A | $ | |||||||||||
| Series A-1 | ||||||||||||
| Series B | ||||||||||||
| Series C | ||||||||||||
| $ | ||||||||||||
Common Stock
The Company has two classes of common stock: Class A common stock and Class B common stock. Class A common stock has
Voting, dividend, and liquidation rights of the holders of the common stock were subject to, and qualified by, the rights, preferences and privileges of the holders of the convertible preferred stock. The holders of the common stock are entitled to
Class A common stock reserved for future issuance consisted of the following:
| September 30, | December 31, | |||||||
| 2024 | 2023 | |||||||
| Convertible preferred stock | ||||||||
| Common stock options granted and outstanding | ||||||||
| Shares available for issuance under the 2024 Plan | ||||||||
| Shares available for issuance under the 2012 Plan | ||||||||
| Preferred stock warrant | ||||||||
| Common stock warrant | ||||||||
| Common stock reserved under the 2024 Employee Stock Purchase Plan | ||||||||
| Total common stock reserved for future issuance | ||||||||
There are
Stock Options
In March 2024, the Company's board of directors and its stockholders adopted and approved the 2024 Equity Incentive Plan (the "2024 Plan"). The 2024 Plan is the successor of the Company's 2012 Equity Incentive Plan (the "2012 Plan"). However, awards outstanding under the 2012 Plan will continue to be governed by their existing terms. The 2024 Plan allowed for the issuance of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted shares, restricted stock units, and other stock awards to the Company's employees, members of its board of directors, and consultants.
The number of shares initially reserved for issuance under the 2024 Plan was
Under the 2024 Plan, the exercise price for options granted under the 2024 Plan may not be less than
The Company's board of directors (or a committee thereof to which the Company's board of directors has delegated authority) may amend or terminate the 2024 Plan at any time. If the Company's board of directors amends the 2024 Plan, it does not need stockholder approval of the amendment unless required by applicable law, regulation or rules. The 2024 Plan will terminate automatically years after the date when the Company's board of directors adopted the 2024 Plan.
In March 2024, the Company's board of directors and its stockholders adopted and approved the 2024 Employee Stock Purchase Plan (the "2024 ESPP"). The 2024 ESPP became effective as of April 9, 2024. The purpose of the 2024 ESPP is to provide eligible employees with an opportunity to increase their interest in the success of the Company by purchasing shares of Class A common stock from the Company on favorable terms and to pay for such purchases through payroll deductions or other approved contributions. The new payroll deduction rate may be any whole percentage of the participant’s compensation, but not less than
As of September 30, 2024, there were
During the nine months ended September 30, 2024, there were
Stock option activity under the 2024 Plan and 2012 Plan is as follows:
| Options Outstanding | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value (in thousands) | |||||||||||||
| Balance at December 31, 2023 | $ | $ | ||||||||||||||
| Options granted | — | — | ||||||||||||||
| Options exercised | ( | ) | — | — | ||||||||||||
| Options cancelled and forfeited | — | — | ||||||||||||||
| Options expired | — | — | ||||||||||||||
| As of September 30, 2024 | $ | $ | ||||||||||||||
| Options vested and expected to vest as of September 30, 2024 | $ | $ | ||||||||||||||
| Options exercisable as of September 30, 2024 | $ | $ | ||||||||||||||
The aggregate intrinsic value of options exercised during the three months ended September 30, 2024 and 2023 was $
The Company estimated the fair value of stock options using the Black-Scholes valuation model. The Company accounts for any forfeitures of options when they occur. Previously recognized compensation expense for an award is reversed in the period that the award is forfeited. The fair value of stock options was estimated using the following weighted-average assumptions:
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| Assumptions: | ||||||||||||||||
| Expected term (in years) | ||||||||||||||||
| Expected volatility | % | % | % | % | ||||||||||||
| Risk free interest rate | % | % | % | % | ||||||||||||
| Dividend yield | ||||||||||||||||
The weighted-average grant-date fair value per share of stock options granted during the three and nine months ended September 30, 2024 was $
The Company recorded $
The Company recorded $
As of September 30, 2024, there was approximately $
7. Income Taxes
The Company’s interim income tax provision consists of U.S. federal and state income taxes based on the estimated annual effective tax rate that the Company expects for the full year together with the tax effect of discrete items. Each quarter the Company updates its estimate of the annual effective tax rate and records cumulative adjustments as necessary.
For the three and nine months ended September 30, 2024, the Company did not record a U.S. federal or state income tax provision due to current and expected annual net operating losses for the year ended December 31, 2024.
As of September 30, 2023, the estimated annual effective tax rate for 2023, exclusive of discrete items, was approximately
Under Section 382 and 383 of the Internal Revenue Code ("IRC"), if a corporation undergoes an ownership change (generally defined as a greater than 50% change in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. The Company has completed an ownership change analysis pursuant to IRC Section 382 for the periods prior to February 9, 2021. On July 13, 2012, April 29, 2018, March 15, 2019, and February 9, 2021, the Company experienced ownership changes. Accordingly, the Company’s ability to utilize net operating loss and tax credit carryforwards attributable to periods prior to February 9, 2021, is subject to substantial limitations. An ownership change analysis pursuant to IRC Section 382 has not been performed for the periods after February 9, 2021, and therefore additional ownership changes may have occurred which may limit the Company’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes.
In assessing the realizability of deferred tax assets, the Company evaluates whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in those periods in which temporary differences become deductible and/or net operating losses can be utilized. The Company assesses all positive and negative evidence when determining the amount of the net deferred tax assets that are more likely than not to be realized. This evidence includes, but is not limited to, prior earnings history, scheduled reversal of taxable temporary differences, tax planning strategies and projected future taxable income. Significant weight is given to positive and negative evidence that is objectively verifiable. Based on these factors, including cumulative losses in recent years, the Company continues to maintain a full valuation allowance against its net deferred tax assets as of September 30, 2024.
8. License Agreement
In February 2023, the Company entered into the J&J License Agreement, pursuant to which the Company granted J&J an exclusive, worldwide license to develop, manufacture and commercialize PIPE-307 in all indications. The J&J License Agreement allows the Company to elect, at its sole discretion and cost, to conduct a Phase 2 trial of PIPE-307 for patients with multiple sclerosis. After such trial, J&J may, at its sole discretion, further develop PIPE-307 for patients with multiple sclerosis. Additionally, upon J&J deciding to conduct a first Phase 3 clinical trial for a product using PIPE-307, the J&J License Agreement allows the Company the option to co-fund a portion of all Phase 3 and subsequent development costs for PIPE-307, with such costs capped annually. If the Company opts to fund such development costs, then the royalties the Company is eligible to receive will increase. Pursuant to the terms of the J&J License Agreement, the Company received an upfront, non-refundable and non-creditable payment of $
The Company sold approximately
The Company concluded that J&J represented a customer and applied relevant guidance from ASC 606 to evaluate the appropriate accounting for the J&J License Agreement. The Company evaluated the J&J License Agreement and concluded that it had promises to transfer a license of functional intellectual property, know-how, existing inventory and manufacturing technology (each of which was determined to be a distinct performance obligation). Control of the promised goods was transferred to J&J in the second quarter of 2023, and the $
In August 2023, the Company elected to conduct a Phase 2 trial using PIPE-307 for patients with multiple sclerosis, which was considered a contract modification under the accounting guidance that added promised goods or services that are distinct at a price that is below the standalone selling price. Therefore, the Company accounted for the modification as a termination of the existing contract and creation of a new contract. Accordingly, the amount of consideration to be allocated to the remaining performance obligations consists of future contingent milestone-based payments and sales-based royalties, all of which were constrained. The only remaining performance obligation is the promise to conduct the Phase 2 trial, as the other performance obligations had been satisfied prior to the modification date. Accordingly, the variable consideration allocated to the Phase 2 trial will be recognized as the study is completed using a cost-based measure of progress and when the amounts are no longer probable of a significant reversal. As of September 30, 2024, no amounts had been recognized related to the Phase 2 trial as no additional variable consideration has been received subsequent to the contract modification.
9. Net Loss Per Share
The following table sets forth the computation of the basic and diluted net income (loss) per share (in thousands, except share and per share amounts):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2024 |
2023 |
2024 |
2023 |
|||||||||||||
| Numerator, basic: |
||||||||||||||||
| Net income (loss) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| Allocation of earnings to participating preferred stockholders |
||||||||||||||||
| Net income (loss) applicable to common stockholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| Denominator, basic: |
||||||||||||||||
| Weighted-average common shares issued |
||||||||||||||||
| Less: weighted-average unvested common stock issued upon early exercise of stock options |
( |
) | ( |
) | ||||||||||||
| Weighted-average shares used to compute net income (loss) per common share, basic |
||||||||||||||||
| Numerator, diluted: |
||||||||||||||||
| Net income (loss) attributable to common stockholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| Change in fair value of warrant liability |
( |
) | ||||||||||||||
| Change in fair value of put option |
( |
) | ||||||||||||||
| Net income (loss) applicable to common stockholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| Denominator, diluted: |
||||||||||||||||
| Weighted-average shares used to compute net income (loss) per common share, diluted |
||||||||||||||||
| Common stock options |
||||||||||||||||
| Unvested common stock issued upon early exercise of stock options |
||||||||||||||||
| Preferred stock warrant (as converted to common stock) |
||||||||||||||||
| Investor rights and obligations | ||||||||||||||||
| Weighted-average shares used to compute net income (loss) per common share, diluted |
||||||||||||||||
| Net income (loss) per share, basic |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
| Net income (loss) per share, diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ||||||
For the three and nine months ended September 30, 2024, net loss is attributable equally to each share of Class A common stock and Class B common stock and is determined based on the weighted-average number of the respective class of common stock outstanding. Weighted-average common shares include shares of the Company's Class A common stock and Class B common stock. The basic and diluted net loss per share amounts are the same for Class A common stock and Class B common stock.
For the three and nine months ended September 30, 2023, there were no shares of Class B common stock outstanding.
The Company’s potentially dilutive securities, which include convertible preferred stock, common stock options, a common stock warrant, unvested common stock issued upon early exercise of stock options, common stock reserved under the 2024 ESPP, and a preferred stock warrant have been excluded from the computation of diluted net loss per share for the three and nine months ended September 30, 2024 and the three months ended September 30, 2023, as the effect would reduce the net loss per share. Therefore, the weighted-average number of shares of common stock outstanding used to calculate both basic and diluted net loss per share attributable to common stockholders is the same.
The following potentially dilutive securities have been excluded from the diluted per share calculation for the periods presented as they would be anti-dilutive:
| Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||
| 2024 |
2023 |
2024 | 2023 | |||||||||||||
| Convertible preferred stock (as converted to common stock) | ||||||||||||||||
| Common stock options |
||||||||||||||||
| Common stock warrant |
||||||||||||||||
| Unvested common stock issued upon early exercise of stock options | ||||||||||||||||
| Common stock reserved under the 2024 ESPP |
||||||||||||||||
| Preferred stock warrant (as converted to common stock) | ||||||||||||||||
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our unaudited financial statements and related notes included in this Quarterly Report on Form 10-Q and the audited condensed financial statements and notes thereto as of and for the year ended December 31, 2023 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations, both of which are included in our final prospectus filed with the Securities and Exchange Commission (“SEC”) pursuant to Rule 424(b) under the Securities Act of 1933, as amended (“Securities Act”) on April 8, 2024 (“Prospectus”) that forms a part of our registration statement on Form S-1 (File No. 333-278003).
This Quarterly Report on Form 10-Q may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Such forward-looking statements, which represent our intent, belief, or current expectations, involve risks and uncertainties. We use words such as “may,” “will,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “predict,” “potential,” “believe,” “should” and similar expressions to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements may include, but are not limited to, statements concerning projections about our accounting and finances, our clinical trial and product development plans and timelines, the indications, anticipated benefits of, and market opportunities for our drug candidates, our operating runway, our business strategies and plans, and other statements regarding future performance. Although we believe the expectations reflected in these forward-looking statements are reasonable, such statements are inherently subject to risk and we can give no assurances that our expectations will prove to be correct. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Quarterly Report on Form 10-Q. As a result of many factors, including without limitation those set forth under “Risk Factors” under Item 1A of Part II below, and elsewhere in this Quarterly Report on Form 10-Q, our actual results may differ materially from those anticipated in these forward-looking statements. We undertake no obligation to update these forward-looking statements to reflect events or circumstances after the date of this report or to reflect actual outcomes.
Overview
We are a clinical stage biopharmaceutical company focused on discovering and developing novel, oral small molecule therapies that target biological pathways associated with specific clinical impairments for the treatment of neuroscience, inflammation and immunology (“NI&I”) indications with high unmet need.
We have focused our efforts on developing selective compounds targeting challenging molecular pathways, and through these efforts, have built a portfolio of small molecule drug candidates.
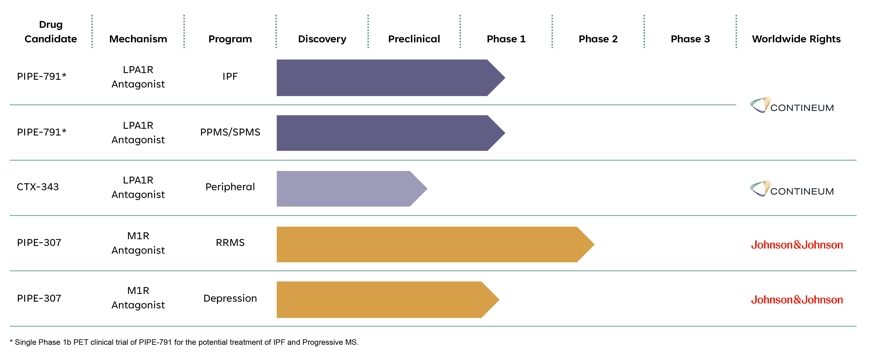
Our wholly-owned lead asset, PIPE-791, is a novel, brain penetrant, small molecule inhibitor of lysophosphatidic acid 1 receptor (“LPA1R”) in development for idiopathic pulmonary fibrosis (“IPF”) and Progressive multiple sclerosis (“Progressive MS”). LPA1R antagonism is a clinically validated mechanism, and we believe that our preclinical studies and Phase 1 healthy volunteer data support the continued development of PIPE-791 for both IPF and Progressive MS. Specifically, based on its high bioavailability, low plasma protein binding, and long receptor residence time in our preclinical studies compared to the preclinical data of other LPA1R antagonists that we know are currently in development, we also believe PIPE-791 has the potential to be a differentiated LPA1R therapy. We completed a Phase 1 clinical trial of PIPE-791 in healthy volunteers in support of clinical development in both IPF and Progressive MS. In September 2024, we submitted a clinical trial application ("CTA") to the Medicines and Healthcare products Regulatory Agency ("MHRA") to commence a Phase 1b open-label trial of PIPE-791 to measure the relationship of pharmacokinetics to lung and brain receptor occupancy by positron emission tomography imaging ("PET"). This Phase 1b trial will inform dose selection for our planned future Phase 2 trials of PIPE-791 in IPF and Progressive MS.
Our second drug candidate, PIPE-307, is a novel, small molecule selective inhibitor of the muscarinic type 1 M1 receptor (“M1R”), in development for depression and relapse-remitting multiple sclerosis (“RRMS”). M1R antagonism has been clinically validated in third-party trials in both depression and RRMS by scopolamine and clemastine, respectively. We have completed two Phase 1 trials of PIPE-307 in healthy volunteers, and we are conducting a Phase 2 trial of PIPE-307, which we have named VISTA, for the potential treatment of RRMS. To our knowledge, PIPE-307 is the most clinically advanced selective M1R antagonist in development. We are developing PIPE-307 in collaboration with Johnson & Johnson ("J&J").
In addition, we are leveraging our drug discovery capabilities synergistically with our clinical portfolio. In January 2024, we nominated and commenced preclinical studies for CTX-343, a peripherally-restricted (unable to cross the blood brain barrier) LPA1R antagonist. In parallel, we are actively conducting preclinical and discovery-phase experiments targeting other NI&I indications where our internally-discovered molecules may have therapeutic potential.
We are currently focused on developing the following product candidates in our pipeline:

We expect our operating expenses to significantly increase as we continue to develop, conduct clinical trials, and seek regulatory approvals for our drug candidates, engage in other research and development activities to expand our pipeline of drug candidates, expand our operations and headcount, maintain and expand our intellectual property portfolio, and, if we obtain approval for one or more of our drug candidates, launch commercial activities. We also expect to incur additional operating expenses as we continue operating as a public company. Our net losses may fluctuate significantly from quarter-to-quarter and year-to-year, depending on the timing and scope of our clinical trials and our expenditures on other research and development activities.
As we continue to pursue our business plan, we expect to finance our operations through both public and private sales of equity, debt financings or other commercial arrangements, which could include income from collaborations, strategic partnerships or marketing, distribution, licensing or other strategic arrangements with third parties. However, there can be no assurance that any additional financing or strategic transactions will be available to us on acceptable terms, if at all. If events or circumstances occur such that we do not obtain additional funding, we may need to delay, reduce or eliminate our product development or future commercialization efforts, which could have a material adverse effect on our business, results of operations or financial condition. Further, if we raise funds through licensing or other commercial arrangements with third parties, we may be required to relinquish valuable rights to our technology, future revenue streams, research programs or drug candidates or may be required to grant licenses on terms that may not be favorable to us and/or may reduce the value of our common stock.
Collaboration
In February 2023, we entered into the license agreement with J&J (the "J&J License Agreement"), pursuant to which we granted J&J an exclusive, worldwide license to develop, manufacture and commercialize PIPE-307 in all indications.
J&J is generally responsible for all development, manufacturing and commercialization activities for PIPE-307. Upon J&J conducting a first Phase 3 clinical trial for a product using PIPE-307, we have an opt-in right to fund a portion of all Phase 3 and subsequent development costs for PIPE-307. If we opt to fund such development costs, then the royalties we are eligible to receive will increase by one to two percentage points.
We are conducting, at our own expense, a Phase 2 clinical trial of PIPE-307 in patients with RRMS. J&J has the right to discontinue our clinical trial if it has good faith concerns that this trial presents safety risks or could have a material adverse effect on its development or commercialization of PIPE-307. In addition, J&J has the right, in its sole discretion, to further develop or to elect not to develop PIPE-307 for this indication.
The J&J License Agreement expires on a licensed product-by-product and country-by-country basis upon the last to occur of: (i) the expiration of the last-to-expire licensed patent claim covering the composition of matter of the licensed compound in such licensed product in such country; (ii) the expiration of exclusive marketing rights conferred by a regulatory authority or applicable law (other than patent exclusivity) for such licensed product in such country; or (iii) ten years after the first commercial sale of such licensed product in such country. Either party may terminate the J&J License Agreement in the event of an uncured material breach by the other party or a bankruptcy or insolvency of the other party. J&J may terminate the J&J License Agreement without cause upon prior written notice to us. Upon any termination, all license rights granted to J&J terminate.
Financial Operations Overview
Revenue
We recognize license revenues as identified performance obligations are satisfied or other events occur, specifically related to our J&J License Agreement. Pursuant to the terms of the J&J License Agreement, we received an upfront payment of $50.0 million in May 2023. We are also eligible to receive approximately $1.0 billion in non-refundable, non-creditable milestone payments, pursuant to the terms of the J&J License Agreement. Additionally, we are eligible to receive tiered royalties in the low-double digit to high-teen percent range on net sales of products containing PIPE-307. We do not have any products approved for sale and we have not yet generated any revenue from product sales.
Operating Expenses
Research and Development
Research and development expenses consist primarily of costs incurred for our internal research and development activities.
Direct costs include:
| • |
employee-related expenses, including salaries, related benefits, and travel that can be directly attributable to each research project; |
| • |
expenses incurred in connection with research, laboratory consumables and preclinical studies; |
| • |
expenses incurred in connection with conducting clinical trials, including investigator grants and site payments for time and pass-through expenses and expenses incurred under agreements with clinical research organizations ("CROs"), other vendors or central laboratories and service providers engaged to conduct our trials; |
| • |
the cost of consultants engaged in research and development related services; |
| • |
the cost to manufacture drug products for use in our preclinical studies and clinical trials; and |
| • |
costs related to regulatory compliance. |
Unallocated internal research and development costs include:
| • |
employee-related expenses, including salaries, related benefits, and travel that cannot be directly attributable to a specific research project; |
| • |
stock-based compensation for employees engaged in research and development functions; and |
| • |
facilities, depreciation and other related expenses. |
We expense our research and development costs as they are incurred. We record advance payments for goods or services to be received in the future for use in research and development as prepaid expenses. We then expense the prepaid amounts as the related goods are delivered or the services are performed.
We track outsourced development costs, consultant costs and other external research and development costs such as third-party contract costs relating to manufacturing, clinical trial activities, translational medicine and toxicology activities to specific programs. We allocate employee related costs, including salaries and related benefits based upon the level of effort for each specific project. Certain employee activities that cannot be allocated to any one specific project or management related activities are considered indirect costs. The following table summarizes our research and development expenses for the three and nine months ended September 30, 2024 and 2023. The direct external development program expenses reflect external costs attributable to our clinical development and preclinical programs and personnel costs that can be directly attributed to a development program. The unallocated internal research and development costs include unallocated personnel costs, facility costs, stock-based compensation, laboratory consumables and discovery and research related activities.
|
|
|
|||||||||||||||
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2024 |
2023 |
2024 |
2023 |
|||||||||||||
| (in thousands) |
(in thousands) |
|||||||||||||||
| Direct external development program expense |
||||||||||||||||
| PIPE-791 |
$ | 2,707 | $ | 3,165 | $ | 7,596 | $ | 7,784 | ||||||||
| PIPE-307 |
3,214 | 1,159 | 7,547 | 2,494 | ||||||||||||
| CTX-343 |
567 | 188 | 1,453 | 581 | ||||||||||||
| Others |
1,109 | 812 | 3,456 | 2,560 | ||||||||||||
| Unallocated internal research and development costs |
||||||||||||||||
| Personnel related |
671 | 461 | 1,524 | 1,072 | ||||||||||||
| Stock-based compensation |
922 | 215 | 1,930 | 682 | ||||||||||||
| Facilities costs |
220 | 204 | 675 | 615 | ||||||||||||
| Other |
318 | 295 | 1,226 | 3,803 | ||||||||||||
| Total research and development costs |
$ | 9,728 | $ | 6,499 | $ | 25,407 | $ | 19,591 | ||||||||
Research and development activities are central to our business model. There are numerous factors associated with the successful commercialization of any of our drug candidates, including future clinical trial design and various regulatory requirements, many of which we cannot determine with accuracy at this time based on our stage of development. In addition, future regulatory factors beyond our control may impact our clinical development programs. Drug candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. At this time, we cannot reasonably estimate or know the nature, timing and costs of the efforts that will be necessary to complete the preclinical and clinical development of any of our drug candidates and our costs may increase if we exercise our opt-in right to fund a portion of all Phase 3 and subsequent development costs for PIPE-307 pursuant to the J&J License Agreement. However, we expect that our research and development expenses will increase substantially in connection with our planned preclinical and clinical development activities in the near term and for the foreseeable future.
The successful development of our drug candidates is highly uncertain. This is due to numerous risks and uncertainties, including the following:
| • |
successful completion of preclinical studies and clinical trials; |
| • |
delays in regulators or institutional review boards authorizing us or our investigators to commence or continue our clinical trials; |
| • |
our ability to negotiate agreements with clinical trial sites or CROs; |
| • |
the number of clinical sites included in our clinical trials; |
| • |
raising additional funds necessary to complete clinical development of our drug candidates; |
| • |
obtaining and maintaining patent, trade secret and other intellectual property protection and regulatory exclusivity for our drug candidates; |
| • |
establishing and qualifying manufacturing capabilities for clinical supplies of our drug candidates, whether directly or through qualified third party manufacturers; |
| • |
our ability to receive necessary regulatory approvals from the U.S. Food and Drug Administration and comparable governmental bodies outside the United States; |
| • |
our decision to elect to fund a portion of Phase 3 and subsequent development costs for PIPE-307; |
| • |
coverage for our products by governmental and third party payors; |
| • |
protecting and enforcing our rights in our intellectual property portfolio; |
| • |
our ability to successfully compete with our competitors and their product offerings; and |
| • |
maintaining a continued acceptable safety profile of the products following approval. |
A change in the outcome of any of these variables with respect to the development of our drug candidates may significantly impact the costs and timing associated with the development of our drug candidates. We may never succeed in obtaining regulatory approval for any of our drug candidates or successfully commercialize our products, even if approved.
General and Administrative
General and administrative expenses consist primarily of salaries and employee-related costs, including stock-based compensation, for personnel in executive, finance and other administrative functions. Other significant costs include legal fees relating to intellectual property, patent applications, and corporate matters, professional fees for accounting and consulting services and facility-related costs.
We expect our general and administrative expenses will increase for the foreseeable future to support our increased research and development activities, the growth of our business operations and headcount and to reflect increased operating expenses as we continue operating as a public company. These increased costs will likely include increased expenses related to audit, legal, regulatory services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations costs.
Other Income (Expense)
Interest Income
Interest income consists of interest earned on our cash, cash equivalents and marketable securities.
Interest Expense
Interest expense consists of (i) interest on our outstanding loan and security agreement (the "Loan Agreement") with First Citizens Bank ("First Citizens"), and (ii) amortization of our debt discount associated with the Loan Agreement recorded in connection with the fair value of the warrant issued to First Citizens, the debt issuance costs incurred and the obligation to make a final payment fee. We repaid all of the outstanding principal on the Loan Agreement as of June 2023.
Results of Operations
Comparison of the Three Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations (in thousands) for the periods indicated:
|
|
||||||||||||
| Three Months Ended September 30, |
||||||||||||
| 2024 |
2023 |
Change |
||||||||||
| Revenue: |
||||||||||||
| License revenue |
$ | — | $ | — | $ | — | ||||||
| Operating expenses: |
||||||||||||
| Research and development |
9,728 | 6,499 | 3,229 | |||||||||
| General and administrative |
3,246 | 1,570 | 1,676 | |||||||||
| Total operating expenses |
12,974 | 8,069 | 4,905 | |||||||||
| Loss from operations |
(12,974 | ) | (8,069 | ) | (4,905 | ) | ||||||
| Other income (expense): |
||||||||||||
| Interest income |
2,741 | 1,736 | 1,005 | |||||||||
| Other expense, net |
(34 | ) | (36 | ) | 2 | |||||||
| Total other income |
2,707 | 1,700 | 1,007 | |||||||||
| Loss before income taxes |
(10,267 | ) | (6,369 | ) | (3,898 | ) | ||||||
| Benefit from income taxes |
— | (118 | ) | 118 | ||||||||
| Net loss |
$ | (10,267 | ) | $ | (6,251 | ) | $ | (4,016 | ) | |||
Research and development expenses. Research and development expenses were $9.7 million and $6.5 million for the three months ended September 30, 2024 and 2023, respectively. The increase of $3.2 million from period to period was primarily due to a $1.1 million increase in contract research organization costs primarily related to our ongoing VISTA Phase 2 clinical trial for PIPE-307 for the potential treatment of RRMS, a $1.5 million increase in expenses for toxicology studies primarily for PIPE-791, a $0.6 million increase in personnel-related expense, and a $0.7 million increase in stock-based compensation. Partially offsetting these increases was a $0.7 million decrease in manufacturing expenses for PIPE-791.
General and administrative expenses. General and administrative expenses were $3.3 million and $1.6 million for the three months ended September 30, 2024 and 2023, respectively. The increase of $1.7 million was primarily due to a $0.2 million increase in consulting and legal expenses, a $1.1 million increase in stock-based compensation, and a $0.3 million increase in personnel-related expenses.
Interest income. Interest income was $2.7 million and $1.7 million for the three months ended September 30, 2024 and 2023, respectively. The increase of $1.0 million was due to an increase in funds invested in marketable securities and an increase in the yields earned on our marketable securities from the three months ended September 30, 2023 to the three months ended September 30, 2024.
Comparison of the Nine Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations (in thousands) for the periods indicated:
|
|
||||||||||||
| Nine Months Ended September 30, |
||||||||||||
| 2024 |
2023 |
Change |
||||||||||
| Revenue: |
||||||||||||
| License revenue |
$ | — | $ | 50,000 | $ | (50,000 | ) | |||||
| Operating expenses: |
||||||||||||
| Research and development |
25,407 | 19,591 | 5,816 | |||||||||
| General and administrative |
8,440 | 4,656 | 3,784 | |||||||||
| Total operating expenses |
33,847 | 24,247 | 9,600 | |||||||||
| Income (loss) from operations |
(33,847 | ) | 25,753 | (59,600 | ) | |||||||
| Other income (expense): |
||||||||||||
| Interest income |
6,377 | 2,816 | 3,561 | |||||||||
| Interest expense |
— | (208 | ) | 208 | ||||||||
| Change in fair value of warrant liability |
(107 | ) | 2 | (109 | ) | |||||||
| Change in fair value of investor rights and obligations liability |
— | 2,867 | (2,867 | ) | ||||||||
| Other expense, net |
(116 | ) | (130 | ) | 14 | |||||||
| Total other income |
6,154 | 5,347 | 807 | |||||||||
| Income (loss) before income taxes |
(27,693 | ) | 31,100 | (58,793 | ) | |||||||
| Provision for income taxes |
— | 611 | (611 | ) | ||||||||
| Net income (loss) |
$ | (27,693 | ) | $ | 30,489 | $ | (58,182 | ) | ||||
License revenue. There was no revenue recognized for the nine months ended September 30, 2024. License revenues were $50.0 million for the nine months ended September 30, 2023 and was solely attributable to the upfront payment from J&J pursuant to the J&J License Agreement.
Research and development expenses. Research and development expenses were $25.4 million and $19.6 million for the nine months ended September 30, 2024 and 2023, respectively. The increase of $5.8 million was primarily due to a $4.1 million increase in contract research organization costs primarily related to our ongoing VISTA Phase 2 clinical trial for PIPE-307 for the potential treatment of RRMS and our completed Phase 1 healthy volunteer clinical trial for PIPE-791, a $3.4 million increase in expenses for toxicology studies primarily for PIPE-791, a $1.7 million increase in personnel-related expense, and a $1.2 million increase in stock-based compensation. Partially offsetting these increases was a $3.0 million decrease in consulting expense associated with contractual milestone payments related to the receipt of the upfront payment from the J&J License Agreement and a $2.4 million decrease in manufacturing expenses for PIPE-791.
General and administrative expenses. General and administrative expenses were $8.4 million and $4.7 million for the nine months ended September 30, 2024 and 2023, respectively. The increase of $3.8 million was primarily due to a $1.3 million increase in consulting and legal expenses, a $1.8 million increase in stock-based compensation, and a $0.5 million increase in personnel-related expenses.
Interest income. Interest income was $6.4 million and $2.8 million for the nine months ended September 30, 2024 and 2023, respectively. The increase of $3.6 million was due to an increase in funds invested in marketable securities and an increase in the yields earned on our marketable securities from the nine months ended September 30, 2023 to the nine months ended September 30, 2024.
Change in fair value of investor rights and obligations liability. We recognized a $2.9 million gain related to the decrease in fair value of our investor rights and obligations liability for the nine months ended September 30, 2023 as a result of reducing the Series B convertible preferred stock premium liability to zero. This was the result of the termination of certain rights due to the change in a specified limited partner’s status in May 2023.
Provision for income taxes. For the nine months ended September 30, 2024, the Company did not record a U.S. federal or state income tax provision due to forecasted pretax operating losses for the year ended December 31, 2024. For the nine months ended September 30, 2023, the Company recorded a tax expense of $0.6 million on pretax income of $31.1 million.
Liquidity and Capital Resources
Sources of Liquidity
We have incurred net losses and negative cash flows from operations in nearly every reporting period since our inception and anticipate that we will continue to incur net losses for the foreseeable future. We expect to incur substantial expenditures as we advance our drug candidates through clinical development, undergo the regulatory approval process, engage in other research and development activities to expand our pipeline of drug candidates, expand our operations and headcount, maintain and expand our intellectual property portfolio and, if we obtain approval for one or more of our drug candidates, launch commercial activities. We also expect to incur additional costs associated with our operating as a public company, including significant legal, accounting, investor relations, director and officer insurance, and other expenses that we did not incur as a private company.
Through September 30, 2024, we have funded our operations primarily through the issuance of convertible promissory notes, the private placements of our convertible preferred stock, the J&J License Agreement, our Loan Agreement with First Citizens, and net proceeds from our initial public offering ("IPO"). Through September 30, 2024, we have raised gross proceeds of approximately $312.8 million from the issuance of our convertible preferred stock, promissory notes, our Class A common stock in the IPO, and have received an upfront payment from the J&J License Agreement of $50.0 million. Upon the closing of the IPO, the Company’s outstanding convertible preferred stock automatically converted into Class A common stock or Class B common stock, as applicable. In April 2024, we raised approximately $107.9 million in net proceeds from the IPO of our shares of Class A common stock. As of September 30, 2024, we had an accumulated deficit of $102.8 million.
As of September 30, 2024, we had cash, cash equivalents and marketable securities of $213.9 million. Management believes our existing cash, cash equivalents, and marketable securities will be sufficient to support our operations for at least 12 months from the date of this Quarterly Report on Form 10-Q.
As we continue to pursue our business plan, we expect to finance our operations through both public and private sales of equity, debt financings or other commercial arrangements, which could include milestone payments from collaborations, strategic partnerships or marketing, distribution, licensing or other strategic arrangements with third parties. However, there can be no assurance that any additional financing or strategic transactions will be available to us on acceptable terms, if at all. If events or circumstances occur such that we do not obtain additional funding, we may need to delay, reduce or eliminate our product development or future commercialization efforts, which could have a material adverse effect on our business, results of operations or financial condition. Further, if we raise funds through licensing or other commercial arrangements with third parties, we may be required to relinquish valuable rights to our technology, future revenue streams, research programs or drug candidates or may be required to grant licenses on terms that may not be favorable to us and/or may reduce the value of our common stock.
Cash Flows
The following table sets forth a summary of our cash flows for the periods indicated (in thousands):
|
|
||||||||
| Nine Months Ended September 30, |
||||||||
| 2024 |
2023 |
|||||||
| Net cash provided by (used in) operating activities |
$ | (22,643 | ) | $ | 30,053 | |||
| Net cash used in investing activities |
(60,351 | ) | (74,598 | ) | ||||
| Net cash provided by financing activities |
108,359 | 56,497 | ||||||
| Net increase in cash and cash equivalents |
$ | 25,365 | $ | 11,952 | ||||
Operating Activities
Net cash used in operating activities was $22.6 million for the nine months ended September 30, 2024, which primarily related to our net loss of $27.7 million, partially offset by $2.6 million of non-cash charges for stock-based compensation, depreciation and amortization, accretion of premiums/discounts on investments, and non-cash operating lease expense and a $2.4 million change in operating assets and liabilities. Net cash provided by operating activities was $30.1 million for the nine months ended September 30, 2023, which primarily related to our net income of $30.5 million and a $1.8 million change in operating assets and liabilities, partially offset by $2.2 million of non-cash charges such as accretion of debt discount and debt issuance costs, stock-based compensation, depreciation and amortization, accretion of premiums/discounts on investments, and non-cash operating lease expense.
Investing Activities
Net cash used in investing activities was $60.4 million for the nine months ended September 30, 2024, which primarily related to $172.1 million of purchases of marketable securities and $0.3 million of purchases of property and equipment, partially offset by $112.0 million of sales and maturities of marketable securities. Net cash used in investing activities was $74.6 million for the nine months ended September 30, 2023, which primarily related to $119.9 million of purchases of marketable securities and $0.1 million of purchases of property and equipment, partially offset by $45.4 million of sales and maturities of marketable securities.
Financing Activities
Net cash provided by financing activities was $108.4 million for the nine months ended September 30, 2024, which primarily related to $107.9 million of net proceeds from the issuance of common stock in the Company's IPO and $0.1 million of proceeds from the exercise of stock options. Net cash provided by financing activities was $56.5 million for the nine months ended September 30, 2023, which primarily related to $60.1 million of net proceeds from the issuance of Series C convertible preferred stock and $0.1 million of proceeds from the exercise of stock options, partially offset by $3.8 million of principal payments on debt.
Funding Requirements
We expect our operating expenses to significantly increase as we continue to develop and seek regulatory approvals for our drug candidates, engage in other research and development activities to expand our pipeline of drug candidates, expand our operations and headcount, maintain and expand our intellectual property portfolio, and, if we obtain approval for one or more of our drug candidates, launch commercial activities. Management believes our existing cash, cash equivalents, and marketable securities will be sufficient to support our operations for at least 12 months from the date of this Quarterly Report on Form 10-Q. However, our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and our actual results could vary materially. We have based this estimate on assumptions that may prove to be wrong, and we could deplete our capital resources sooner than we expect. Additionally, the process of testing our drug candidates in clinical trials is costly, and the timing of progress and expenses in these trials is uncertain.
Our future capital requirements will depend on many factors, including:
| • |
the type, number, scope, progress, expansions, results, costs and timing of, our clinical trials and preclinical studies for our drug candidates or other potential drug candidates or indications which we are pursuing or may choose to pursue in the future; |
| • |
the outcome, timing and costs of regulatory review of our drug candidates; |
| • |
the costs and timing of manufacturing for our drug candidates; |
| • |
our efforts to enhance our operational systems and hire additional personnel to satisfy our obligations as a public company, including enhanced internal controls over financial reporting; |
| • |
the costs associated with hiring additional personnel and consultants as our preclinical and clinical activities expand; |
| • |
the costs and timing of establishing or securing manufacturing facilities for our drug candidates; |
| • |
the costs and timing of establishing sales and marketing capabilities if any of our drug candidates are approved; |
| • |
our ability to establish and maintain strategic collaborations, licensing or other arrangements; |
| • |
the financial terms of any such agreements that we may enter into; |
| • |
our decision to elect to fund a portion of Phase 3 and subsequent development costs for PIPE-307; |
| • |
the costs of obtaining, maintaining and enforcing our patent and other intellectual property rights; and |
| • |
costs associated with any drug candidates, products or technologies that we may in-license or acquire. |
Until such time as we can generate significant revenue from sales of our drug candidates, if ever, we expect to finance our cash needs through public or private equity or debt financings or other commercial arrangements, including collaborations, strategic partnerships or marketing, distribution, licensing or other strategic arrangements with third parties. We may be unable to raise additional funds or enter into such commercial arrangements when needed, on favorable terms, or at all. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be or could be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing and equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, or other similar arrangements with third parties, we may be required to relinquish valuable rights to our drug candidates, future revenue streams or research programs or may be required to grant licenses on terms that may not be favorable to us and may reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings or through commercial arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market our drug candidates even if we would otherwise prefer to develop and market such drug candidates ourselves.
Contractual Obligations and Commitments
Our contractual obligations and commitments relate to our operating leases that relate primarily to our leases of office and laboratory space in San Diego, California. Our total contractual commitments for our lease agreements amount to approximately $7.8 million as of September 30, 2024.
We enter into contracts in the normal course of business for contract research services, contract manufacturing services, professional services and other services and products for operating purposes. These contracts generally provide for termination after a notice period, and, therefore, are cancelable contracts and not included in the table above.
Off-Balance Sheet Arrangements
During the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under the rules and regulations of the SEC.
Critical Accounting Policies and Significant Judgments and Estimates
Our discussion and analysis of financial condition and results of operations are based upon our condensed financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make significant estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses and related disclosures. On an ongoing basis, our actual results may differ significantly from our estimates.
There have been no material changes to our critical accounting estimates from those described under our “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Critical Accounting Policies and Significant Judgements and Estimates” included in our Prospectus, except that from the effectiveness date of our registration statement on Form S-1 (File No. 333-278003), our common stock is publicly traded and we therefore no longer require common stock valuations.
Emerging growth company and smaller reporting company status
We are an “emerging growth company”, as defined in the Jumpstart Our Business Startups Act of 2012 (the "JOBS Act"). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. We will remain an emerging growth company until the earlier of (i) December 31, 2029, the last day of the fiscal year following the fifth anniversary of the completion of the Company's IPO, (ii) the last day of the fiscal year (a) in which we have total annual gross revenue of at least $1.235 billion or (b) in which we are deemed to be a large accelerated filer, which means the market value of our voting and non-voting common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th, or (iii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as the market value of our voting and non-voting common stock held by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our voting and non-voting common stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and our principal financial officer, evaluated, as of the end of the period covered by this Quarterly Report on Form 10-Q, the effectiveness of our disclosure controls and procedures. Based on this evaluation of our disclosure controls and procedures as of September 30, 2024, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures as of such date were effective at the reasonable assurance level. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act are recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Control over Financial Reporting
During the quarter ended September 30, 2024, there have been no changes in our internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15(d)-15(f) promulgated under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
From time to time, we may become involved in various legal proceedings and claims that arise in the ordinary course of our business activities. We are not currently a party to any material legal proceedings. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, negative publicity, reputational harm and other factors.
Investing in our common stock involves a high degree of risk. In addition to the information set forth in this Quarterly Report on Form 10-Q, including the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes, you should consider carefully the factors discussed in Part I, Item 1A, “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 filed with the Securities and Exchange Commission on May 16, 2024. The occurrence of any of the risks and uncertainties described in such Quarterly Report could materially and adversely affect our business, financial condition, results of operations and prospects. In that event, the price of our common stock could decline and you could lose part or all of your investment. Furthermore, such risks are not the only ones we face; additional risks and uncertainties not currently known or that we currently deem to be immaterial may also materially adversely affect our business, financial condition or results of operations.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
(a) Recent Sales of Unregistered Equity Securities.
None.
(b) Use of Proceeds from Initial Public Offering of Common Stock.
On April 4, 2024, our registration statement on Form S-1 (333-278003) relating to the initial public offering of our common stock was declared effective by the SEC (the "Registration Statement"). Pursuant to such Registration Statement, we issued and sold an aggregate of 7,423,682 shares of our common stock, which includes 548,682 shares sold pursuant to the underwriters’ partial exercise of their option to purchase additional shares, at the public offering price of $16.00 per share. On April 9, 2024, we closed the sale of 6,875,000 shares and on April 19, 2024, we closed the sale of the 548,682 shares sold pursuant to the underwriters’ exercise of their option to purchase additional shares. The aggregate offering price for shares sold in the IPO was approximately $118.8 million, resulting in aggregate net proceeds of approximately $107.9 million, after deducting the underwriting discounts, commissions and offering expenses paid or payable by us. None of the expenses associated with the IPO were paid to directors, officers, persons owning 10% or more of any class of equity securities, or to our affiliates. Goldman Sachs & Co. LLC, Morgan Stanley, Stifel and RBC Capital Markets acted as joint book-running managers for the IPO.
There has been no material change in the planned use of proceeds from the IPO from those described in the Prospectus, dated April 4, 2024, filed with the SEC on April 8, 2024, pursuant to Rule 424(b) of the Securities Act.
(c) Issuer Purchases of Equity Securities.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not Applicable.
From time to time, our officers (as defined in Rule 16a-1(f) of the Exchange Act) and directors may enter into Rule 10b5-1 or non-Rule 10b5-1 trading arrangements (as each such term is defined in Item 408 of Regulation S-K). During the three months ended September 30, 2024, none of our officers or directors adopted or terminated any such trading arrangements except as set forth below:
| Name (Title) | Action Taken (Date of Action) | Type of Trading Arrangement | Nature of Trading Arrangement | Duration of Trading Arrangement | Aggregate Number of Securities |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
|
| Adoption ( ) | Rule - trading arrangement* | Sale | Until , or such earlier date upon which all transactions are completed or expire without execution | Up to |
* Intended to satisfy the affirmative defense of Rule 10b5-1(c)
| Incorporated by Reference |
Filed Herewith |
||||||
| Exhibit |
Description |
Form |
File No. |
Exhibit |
Filing Date |
||
| 3.1 |
Amended and Restated Certificate of Incorporation of the Registrant. |
8-K |
001-42001 |
3.1 |
04/09/2024 |
||
| 3.2 |
8-K |
001-42001 |
3.2 |
04/09/2024 |
|||
| 31.1 |
X |
||||||
| 31.2 |
X |
||||||
| 32.1* |
X |
||||||
| 32.2* |
X |
||||||
| 101.INS |
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
X |
|||||
| 101.SCH |
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents. |
X |
|||||
| 104 |
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
X |
_________________________________________
* The certifications furnished in Exhibit 32.1 and 32.2 hereto are deemed to be furnished with this Quarterly Report on Form 10-Q and will not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, except to the extent that the Registrant specifically incorporates them by reference.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Contineum Therapeutics, Inc. |
|||
| November 6, 2024 |
By: |
/s/ Carmine Stengone |
|
| Carmine Stengone |
|||
| President and Chief Executive Officer |
|||
| (Principal Executive Officer) | |||
|
|
|||
| November 6, 2024 |
By: |
/s/ Peter Slover |
|
| Peter Slover |
|||
| Chief Financial Officer |
|||
| (Principal Financial Officer and Principal Accounting Officer) | |||