團結起來 各州
證券交易委員會
華盛頓, 直流電 20549
表格
(馬克 一)
| 根據1934年證券交易法第13或15(d)節提交的季度報告書 |
截至季度結束
或者
| 根據1934年證券交易法第13或15(d)節提交的過渡報告書 |
對於從_________到_________的過渡期
委員會
文件編號:
(符合其章程規定的註冊人的確切名稱)
(或其他轄區 (組織)的註冊地點 |
(國稅局僱主 (主要 執行人員之地址) | |
| (主要 執行人員之地址) | (郵政 編 碼) |
根據交易所法規(17 CFR 240.14a-12)第14a-12規定的招股材料
不是 適用的
(公司更名、更改地址和更改財年情況的以往名稱、以前地址和以前財年,如與上次報告有所改變)
根據法案第12(b)節註冊的證券:
| 每一類別的名稱 | 交易符號 | 在每個交易所註冊的名稱 | ||
| 這 |
請勾選標記以指示註冊者是否(1)在過去12個月內(或註冊者需要提交這些報告的更短時間內)已提交證券交易所法案第13或15(d)節要求提交的所有報告,及 (2)是否已被提交要求過去90天的提交要求所制約。
請在勾選框內勾選,以指示註冊人在過去的12個月內(或註冊人需要提交這些文件的時間更短)是否已經電子提交了每一份互動數據文件,該提交是根據證券法規定第405條規則和本章第232.405條規則規定。
請勾選以下選項以確認該註冊申請人是否爲大型快速提交者、快速提交者、非加速提交者、小型報告公司或新興成長型公司。請參閱交易所法規120億.2中「大型快速提交者」、「快速提交者」、「小型報告公司」和「新興成長型公司」的定義。
| 大型加速文件提交人 | ☐ | 加速文件提交人 | ☐ | |
| ☒ | 小型報告公司 | |||
| 新興成長公司 | ||||
如果是新興成長公司,請勾選,如果註冊人已選擇不使用根據交易所法案第13(a)條提供的任何新的或修改的財務會計準則的延長過渡期,請勾選。
請勾選適用的圓圈,表示註冊登記者是否是空殼公司(根據交易所法案第12b-2條的定義)。是 ☐ 否
2024年11月12日,普通股流通股數爲 .
頁面 不。 | ||
| 第一部分 財務信息 | ||
| 項目1。 | 基本報表 | 4 |
| 2024年9月30日(未經審計)和2023年12月31日的簡明合併資產負債表 | 4 | |
| 2024年9月30日和2023年3個月及9個月未經審計的壓縮合並利潤表和綜合虧損表 | 5 | |
| 2024年9月30日結束的三個月和九個月的未經審計的基本財務報表股東權益表 | 6 | |
| 2024年9月30日結束的九個月的未經審計的基本財務報表現金流量表 | 7 | |
| 基本合併財務報表附註(未經審計) | 8 | |
| 項目 2. | 分銷計劃 | 25 |
| 項目 3. | 有關市場風險的定量和定性披露 | 33 |
| 項目 4. | 控制和程序 | 34 |
| 第二部分.其他信息 | ||
| 項目1。 | 法律訴訟 | 35 |
| Interest expense, net | 風險因素 | 35 |
| 項目 2. | 未註冊的股票股權銷售和籌款用途 | 36 |
| 項目5。 | 其他信息 | 37 |
| 項目 6. | 展示資料 | 37 |
| 簽名 | 38 | |
| 2 |
關於前瞻性聲明和行業數據的注意事項
本季度報告,根據1933年修訂版《證券法》(以下簡稱「證券法」)第27A條和1934年修訂版《證券交易法》(以下簡稱「交易法」)第21E條的安全港規定,包含前瞻性聲明。這些聲明可以通過「可能」、「應當」、「預計」、「打算」、「計劃」、「預期」、「相信」、「估計」、「預測」、「潛在」、「繼續」或其他類似用語識別爲前瞻性術語。我們的前瞻性聲明基於對公司的期望、假設、估計和投影序列,不保證未來結果或績效,並涉及重大風險和不確定性。我們可能無法實際達到這些前瞻性聲明中披露的計劃、意圖或期望,實際結果或事件可能與這些前瞻性聲明中披露的計劃、意圖和期望存在重大差異。我們的業務和前瞻性聲明涉及重大已知風險和不確定性,包括有關我們陳述的風險和不確定性:
| ● | 我們的預期財務狀況和估計的現金燃燒率; | |
| ● | 我們關於費用、未來收入和資本需求的估計; | |
| ● | 我們作爲持續經營的能力; | |
| ● | 我們需要籌集大量額外資本來資助我們的業務,此類資金的可用性和條件,以及由此產生的稀釋; | |
| ● | 我們臨床試驗的成功、成本和時間安排; | |
| ● | 我們在進行臨床試驗過程中對第三方的依賴; | |
| ● | 我們獲得必要的監管批准來營銷和商業化我們的產品候選者的能力; | |
| ● | 健康流行病對我們的業務、臨床試驗、研究項目、醫療系統或整個全球經濟的最終影響; | |
| ● | 通過臨床前和臨床試驗結果表明,我們當前的產品候選者或可能尋求開發的任何未來產品候選者可能不安全或無效; | |
| ● | 我們或其他機構進行的市場調研結果; | |
| ● | 我們獲得和保持對我們當前和未來產品候選者的知識產權保護的能力; | |
| ● | 我們保護知識產權的能力,以及我們因執行或保護知識產權而產生巨大訴訟費用的潛力; |
| ● | 第三方可能聲稱我們或我們的第三方許可人侵犯、侵佔或以其他方式侵犯了他們的知識產權,我們可能會承擔巨大的費用並被要求大量時間來辯護我們自己; | |
| ● | 我們依賴第三方供應商和製造商; | |
| ● | 競爭產品和療法成功的可能性; | |
| ● | 我們能否擴大我們的組織以適應潛在的增長並保留和吸引關鍵人員; | |
| ● | 我們可能會因產品責任訴訟而產生大量費用,這些產品責任訴訟可能導致我們限制產品候選品的商業化。 |
| ● | 市場接受我們的產品候選者,對當前產品候選者和我們可能尋求開發的任何未來產品候選者的潛在市場規模和增長以及我們爲這些市場提供服務的能力;和 | |
| ● | 成功發展我們的商業化能力,包括銷售和營銷能力。 |
所有板塊 我們所有的前瞻性聲明僅截至本季度10-Q表格的日期。在每種情況下,實際結果可能與這些前瞻性信息有實質性差異。我們無法保證這些期望或前瞻性聲明將被證明是正確的。在年終爲2023年12月31日的年度報告10-K中提到的風險因素或風險和不確定性的一個或多個發生,或任何重大不利變化,該報告已於2024年3月29日向證券交易委員會(SEC)提交,本季度的10-Q報告,或包含在我們的其他公開披露或其他定期報告或提交給美國證券交易委員會(「SEC」)的其他文件或申報的一部分可能會對我們的業務,前景,財務狀況和運營結果產生重大不利影響。除非法律要求,我們不會更新或修訂任何這樣的前瞻性聲明,以反映實際結果,計劃變化,假設,估計或其他影響這種前瞻性聲明的情況,即使這樣的結果,變化或情況顯然表明任何前瞻性信息將不會實現。我們在本季度10-Q報告之後發表的任何公開聲明或披露,修改或影響本季度10-Q報告中包含的前瞻性聲明都將被視爲修改或取代本季度10-Q報告中的這些聲明。
本季度10-Q表格中可能包含市場數據和某些行業數據和預測,我們可能從內部公司調查、市場研究、諮詢調查、公開信息、政府機構報告和行業出版物、文章和調查中獲取這些信息。行業調查、出版物、諮詢調查和預測通常聲明其中包含的信息來自可信來源,但不保證這些信息的準確性和完整性。雖然我們相信這些研究和出版物是可靠的,但我們尚未獨立驗證第三方來源的市場和行業數據,也未委託任何此類信息。
| 3 |
部分I—財務信息
項目1. 基本報表。
Immix 生物製藥公司。
簡明合併資產負債表
| 2024年9月30日 | 2023年12月31日 | |||||||
| (未經審計) | ||||||||
| 資產 | ||||||||
| 流動資產: | ||||||||
| 現金及現金等價物 | $ | $ | ||||||
| 應收稅款 | ||||||||
| 預付費用和其他流動資產 | ||||||||
| 總流動資產 | ||||||||
| 其他 | ||||||||
| 待攤股票發行費用 | ||||||||
| 淨使用權資產 | ||||||||
| 房地產和設備,淨額 | ||||||||
| 總資產 | $ | $ | ||||||
| 負債和股東權益 | ||||||||
| 流動負債: | ||||||||
| 應付賬款和應計費用 | $ | $ | ||||||
| 經營租賃負債-流動 | ||||||||
| 流動負債合計 | ||||||||
| 長期經營租債務 | ||||||||
| 總負債 | ||||||||
| 承諾和 contingencies | ||||||||
| 股東權益: | ||||||||
| 優先股,$0.0001 面值; 已授權股份; 已發行和流通的股份數量 | ||||||||
| 普通股,每股面值爲 $0.0001; 面值; 已授權股份; 發行股爲 於2024年9月30日的流通股份和其它 發行股爲 和 | ||||||||
| 額外實收資本 | ||||||||
| 累計其他綜合收益 | ||||||||
| 累積赤字 | ( | ) | ( | ) | ||||
| 成本法下的庫藏股, 截至2024年9月30日和2023年12月31日的股份 | ( | ) | ( | ) | ||||
| Total Immix Biopharma,Inc.股東權益 | ||||||||
| 非控制股權 | ( | ) | ||||||
| 股東權益總額 | ||||||||
| 負債和股東權益總額 | $ | $ | ||||||
請參閱簡明合併財務報表信息附註。
| 4 |
Immix 生物製藥公司。
彙編的綜合損失和營運狀況
(未經審計)
| 截至三個月的情況 | 截至九個月結束 | |||||||||||||||
| 九月三十日, | 九月三十日, | |||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| 營業費用: | ||||||||||||||||
| General and administrative expenses | $ | $ | $ | $ | ||||||||||||
| 研發 | ||||||||||||||||
| 總營業費用 | ||||||||||||||||
| 營運虧損 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| 其他收入(費用): | ||||||||||||||||
| 利息收入 | ||||||||||||||||
| 其他支出合計,淨值 | ||||||||||||||||
| 稅前虧損 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| 所得稅準備金 | ||||||||||||||||
| 淨損失 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| 歸屬於少數股東的淨虧損 | ||||||||||||||||
| Immix生物製藥公司普通股股東應付淨虧損 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| 其他全面收益(損失): | ||||||||||||||||
| 外匯翻譯 | ( | ) | ( | ) | ||||||||||||
| 其他全面損失總額 | ( | ) | ( | ) | ||||||||||||
| 綜合損失 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Less: comprehensive loss attributable to non-controlling interests | ||||||||||||||||
| Immix生物製藥公司普通股股東應付綜合虧損 | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| 每股普通股基本和稀釋的虧損 | $ | ) | $ | ) | $ | ) | $ | ) | ||||||||
| 基本和稀釋後的流通股數平均權重 | ||||||||||||||||
請參閱簡明合併財務報表信息附註。
| 5 |
Immix 生物製藥公司。
壓縮的 股東權益合併報表
2024年和2023年截至9月30日的三個月和九個月
(未經審計)
| 常見 | 額外 | 其他積累 | 國庫 | 非- | 總計 | |||||||||||||||||||||||||||||||
| Common | 股票 | 實收資本 | 綜合 | Accumulated | 國庫 | 股票 | 控制 | 股東的 | ||||||||||||||||||||||||||||
| 股份 | 金額 | 資本 | 收入 | 赤字 | 股份 | 金額 | 利息 | 資產淨值 | ||||||||||||||||||||||||||||
| 2023年12月31日餘額 | $ | $ | $ | $ | ( | ) | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||||||||
| 通過ATM機制發行股票,募集資金淨額扣除發行費用 | - | |||||||||||||||||||||||||||||||||||
| 通過公開發售發行股票,募集資金淨額扣除發行費用 | - | |||||||||||||||||||||||||||||||||||
| 行使股票期權發行的股票 | - | |||||||||||||||||||||||||||||||||||
| 服務發行的股份數 | - | |||||||||||||||||||||||||||||||||||
| 股權補償 | - | |||||||||||||||||||||||||||||||||||
| 子公司非控股權益 | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| 淨損失 | - | ( | ) | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | ( | ) | - | ( | ) | ||||||||||||||||||||||||||||||
| 2024年3月31日餘額 | $ | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||||||||
| 股份發行以換取服務 | - | |||||||||||||||||||||||||||||||||||
| 以股票爲基礎的補償 | - | - | ||||||||||||||||||||||||||||||||||
| 子公司中的非控制權益 | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| 購買子公司中的非控制權益 | ( | ) | - | |||||||||||||||||||||||||||||||||
| 淨損失 | - | ( | ) | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | - | ||||||||||||||||||||||||||||||||||
| 2024年6月30日結餘 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| 股份發行用於服務 | - | |||||||||||||||||||||||||||||||||||
| 股權補償 | - | - | ||||||||||||||||||||||||||||||||||
| 淨損失 | - | ( | ) | - | ( | ) | ||||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | - | ||||||||||||||||||||||||||||||||||
| 2024年9月30日的餘額 | $ | $ | $ | $ | ( | ) | ( | ) | $ | ( | ) | $ | $ | |||||||||||||||||||||||
| 2022年12月31日期末餘額 | $ | $ | $ | $ | ( | ) | ( | ) | $ | ( | ) | $ | $ | |||||||||||||||||||||||
| 通過ATM設施發行股份以獲得現金收益,扣除發行成本淨額 | - | |||||||||||||||||||||||||||||||||||
| Nexcella股份以現金收益發行 | - | - | ||||||||||||||||||||||||||||||||||
| 以股票爲基準的薪酬 | - | |||||||||||||||||||||||||||||||||||
| 子公司中的非控股權益 | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| 淨虧損 | - | ( | ) | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | ( | ) | - | ( | ) | ||||||||||||||||||||||||||||||
| 截至2023年3月31日的餘額 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 以ATm機構發放股份以現金收益,淨扣除發行費用 | - | |||||||||||||||||||||||||||||||||||
| 以股票爲基礎的薪酬 | - | |||||||||||||||||||||||||||||||||||
| 子公司中的非控制權益 | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| 淨損失 | - | ( | ) | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | ( | ) | - | ( | ) | ||||||||||||||||||||||||||||||
| 6月份結餘2023年6月30日 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 依據ATM業務設施發行的股份,扣除發行成本後的現金收益淨額 | - | |||||||||||||||||||||||||||||||||||
| 在定向增發下發行的股份和認股權證,扣除發行成本後的現金收益淨額 | - | |||||||||||||||||||||||||||||||||||
| 以股票爲基礎的補償 | - | - | ||||||||||||||||||||||||||||||||||
| 子公司中的非控制權益 | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| 淨損失 | - | ( | ) | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| 外幣翻譯調整 | - | ( | ) | - | ( | ) | ||||||||||||||||||||||||||||||
| 2023年9月30日結餘 | $ | $ | $ | $ | ( | ) | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||||||||
請參閱未經審計的簡明合併基本報表的附註。
| 6 |
Immix 生物製藥公司
簡明 合併現金流量表
(未經審計)
| 截至九個月 | ||||||||
| 九月三十日 | ||||||||
| 2024 | 2023 | |||||||
| 經營活動: | ||||||||
| 淨損失 | $ | ( | ) | $ | ( | ) | ||
| 調整淨虧損與經營活動使用的現金的折算: | ||||||||
| 基於股票的補償 | ||||||||
| 折舊 | ||||||||
| 使用權資產的攤銷 | ||||||||
| 經營資產和負債的變動: | ||||||||
| 應收稅款 | ( | ) | ( | ) | ||||
| 預付款項及其他流動資產 | ( | ) | ||||||
| 其他資產 | ( | ) | ||||||
| 應付賬款和預提費用 | ||||||||
| 經營租賃負債 | ||||||||
| 淨現金流出活動 | ( | ) | ( | ) | ||||
| 投資活動: | ||||||||
| 購買房地產和設備 | ( | ) | ( | ) | ||||
| 投資活動中使用的淨現金 | ( | ) | ( | ) | ||||
| 融資活動: | ||||||||
| 遞延發行費用的支付 | ( | ) | ||||||
| 普通股銷售淨收益,扣除發行成本 | ||||||||
| 股票期權行使所獲收益 | ||||||||
| 子公司私募融資收到的資金 | ||||||||
| 融資活動提供的淨現金 | ||||||||
| 外幣對現金的影響 | ||||||||
| 現金及現金等價物淨變動額 | ||||||||
| 現金及現金等價物 – 期初餘額 | ||||||||
| 現金及現金等價物 – 期末餘額 | $ | $ | ||||||
| 現金流信息補充披露: | ||||||||
| 已支付的所得稅 | $ | $ | ||||||
| 非現金融資信息的補充披露: | ||||||||
| 使用權資產和負債的建立 | $ | $ | ||||||
| 應付賬款和應計負債中包括的物業和設備採購 | ||||||||
| 遞延發行成本從普通股銷售收入中扣除 | $ | $ | ||||||
| 在子公司吸收中發行的股份 | $ | $ | ||||||
| Nexcella爲之前收到的資金髮行的股份 | $ | $ | ||||||
請參閱未經審計的簡明合併基本報表的附註。
| 7 |
Immix 生物製藥公司
合併財務報表註釋
(未經審計)
注意 1 – 業務性質
Immix 生物製藥有限公司(以下簡稱「公司」)是一家臨床階段的生物製藥公司,成立於2014年1月7日,註冊於特拉華州,專注於開發阿爾茨海默症澱粉樣變性及特定的免疫介導疾病的電芯治療。2016年8月, 公司成立了一家全資子公司,Immix Biopharma Australia Pty Ltd.(「IBAPL」),以進行其開發候選藥物的各種前臨床和臨床活動。2022年11月,公司建立了一家控股子公司,Nexcella, Inc.(「Nexcella」),其電芯治療部門,隨後於2024年5月併入公司, 公司繼續作爲存續實體。爲確保操作的連續性,公司於2024年重新建立Nexcella,作爲全資子公司。
注意 2 – 重要會計政策概述
呈現基礎 - 隨附的簡明合併基本報表及相關附註是按照美國通用會計原則(「U.S. GAAP」)和美國證券交易委員會(「SEC」)的規則和規定編制的。公司的財政年度結束於12月31日。
截至2024年9月30日的凝縮合並基本報表及相關披露,以及截至2024年和2023年9月30日的三個月和九個月數據未經審計,按照SEC的規則和規定。某些通常包含在符合美國通用會計原則編制的基本報表中的信息和腳註披露已根據這些規則和規定進行了簡化或省略。根據公司的意見,這些未經審計的凝縮合並基本報表包含了爲公正陳述中期結果所需的所有調整(僅包括正常的定期調整)。這些未經審計的凝縮合並基本報表應與公司截至2023年和2022年12月31日的審計基本報表一起閱讀,這些基本報表包含在公司於2024年3月29日向SEC提交的10-k表格的年度報告中。截至2024年9月30日的三個月和九個月的經營結果並不一定預示着截至2024年12月31日的完整年度的結果。
風險 和不確定性 - 公司運營於一個動態且競爭激烈的行業,面臨生物技術行業早期公司普遍面臨的風險和不確定性,包括但不限於,競爭對手開發新科技創新,保護專有技術,依賴關鍵人員、代工廠商和醫藥外包概念,遵循政府法規及需要獲得額外融資以支持運營。目前正在開發的產品候選者將需要進行大量額外的研究和開發工作,包括廣泛的臨床前研究、臨床試驗和監管批准,才可以商業化。這些工作需要大量額外資本、足夠的人員基礎設施以及廣泛的合規和報告。公司認爲,以下任何領域的變化都可能對公司的未來財務狀況、經營結果或現金流產生重大不利影響:獲得未來融資的能力;新科技和行業標準的進展和趨勢;臨床試驗結果;公司的產品的監管批准和市場接受度;銷售渠道的開發;某些戰略關係;基於知識產權、專利、產品、監管或其他因素對公司的訴訟或索賠;以及公司吸引和留住支持其增長所需員工的能力。
公司開發的產品在商業銷售之前需要獲得美國食品和藥物管理局(「FDA」)或其他國際監管機構的批准。無法保證公司的研究和開發會順利完成,無法保證獲得或維護足夠的知識產權保護,產品是否會獲得必要的批准,或任何獲得批准的產品是否會在商業上可行。如果公司被拒絕批准,批准被延遲,或公司無法維持批准,可能會對公司產生重大不利影響。即使公司的產品開發工作成功,但公司何時能從產品銷售中獲得營業收入仍不確定。如果公司成功,何時能盈利仍然不確定。公司在科技快速變化和來自其他藥品和生物技術公司的激烈競爭的環境中運營。此外,公司依賴於員工、顧問和其他第三方的服務。
| 8 |
公司已經支出並計劃繼續支出大量資金用於完成產品候選者的研究、開發和臨床測試。公司還需要額外支出資金以建立商業規模的製造業-半導體安排,併爲獲得監管審批的產品提供營銷和分銷。公司可能需要額外資金來實現其產品的商業化。公司無法完全依靠其當前的財務資源來資助這些努力,並將需要在未來籌集額外資金。如果運營或其他融資來源未能及時提供充足資金,公司可能不得不延遲、縮小或取消其一個或多個研究或開發項目,這可能會對其業務、財務狀況和運營產生重大不利影響。
估算的使用 – 按照美國通用會計準則編制這些簡明合併基本報表,管理層需做出估計和假設,以影響財務報表日期的資產和負債的報告金額,以及報告期內收入和費用的報告金額。公司在進行與遞延稅資產及相關估值準備、研發費用的計提和預付款,以及基於股票的薪酬估值相關的估計時,使用了重要的判斷。實際結果可能與這些估計有所不同。
合併原則 – 隨附的簡化合並基本報表包括Immix Biopharma, Inc.及其
分部
報告 - 公司將其運營視爲一個單一板塊,以便進行業績評估和做出運營決策。公司的首席運營決策者(「CODM」)是其首席執行官。CODM根據公司的綜合層面的信息,分配資源並評估公司的業績,這些信息包括其收入、毛利潤、運營收入和其他重要財務數據。所有重要的運營決策都是基於對公司的分析。
流動性
然後繼續關注 – 這些合併財務報表是在持續經營的基礎上編制的,前提是
公司將繼續在正常業務過程中變現資產並清償負債。自從首次公開以來
該公司於2021年12月發行普通股,通過各種股權融資爲其運營融資。7月14日
2023 年,公司與 ThinkEquity LLC 簽訂了額外的自動櫃員機銷售協議(「7 月銷售協議」)(「銷售」)
代理人”),根據該協議,公司可以不時通過銷售代理發行和出售公司的股份
按照《證券》頒佈的第 415 (a) (4) 條的定義,銷售中的普通股被視爲 「市場發行」
經修訂的1933年法案(「7月自動櫃員機設施」)(見註釋7)。最初,公司有資格出售最高美元
從
2023年7月14日到2024年2月5日,公司已售出 普通股,依據七月ATM融資協議,淨收益爲
$
在2024年2月,公司進行了公開承銷股票的發行,
| 9 |
在2024年7月25日,公司獲得了來自加利福尼亞再生醫學研究所的一筆百萬美元的補助金,
本公司歷史上曾出現並預計將繼續報告來自運營的負現金流和淨損失。我們相信,截至2024年9月30日,我們現有的現金、現金等價物和受限現金將使我們能夠在至少未來12個月內資助我們的營業費用和資本支出需求。
信用風險集中 – 定期,公司可能在金融機構持有超過聯邦保險限額的現金及現金等價物餘額$
現金 及現金等價物 公司的現金等價物包括在購買時原始到期日爲90天或更短的短期高流動性投資,按公允價值計量。
金融工具的公允價值 – 短期工具的賬面價值,包括現金及現金等價物、應收稅款、應付賬款和應計費用,由於這些工具的到期期間相對較短,接近公允價值。
公允價值被定義爲在測量日期,市場參與者之間進行有序交易時,資產所接收的交換價格或轉移負債所支付的價格(退出價格),該價格在資產或負債的主要或最有利市場中。 用於衡量公允價值的估值技術最大程度地利用可觀察輸入,並儘量減少不可觀察輸入的使用。公司採用三級估值層次結構來披露公允值測量,定義如下:
層級 1 – 估值方法的輸入是活躍市場中相同資產或負債的報價(未經調整)。
等級 2 – 估值方法的輸入包括在活躍市場中類似資產和負債的報價,以及可觀察到的資產或負債的輸入,無論是直接還是間接,涵蓋金融工具的實質性完整期限。
級別 3 – 估值方法的輸入是不可觀察的,並且對公允價值具有重要影響。
| 10 |
以下公允價值等級表提供了關於公司資產的公允價值定期計量的信息:
| 截至2024年9月30日的公允價值計量 | ||||||||||||
| 一級 | 二級 | 第三級 | ||||||||||
| 資產: | ||||||||||||
| 現金等價物(貨幣市場基金) | $ | $ | $ | |||||||||
截至2024年9月30日,公司擁有
| 截至2023年12月31日的公允價值測量 | ||||||||||||
| 一級 | 二級 | 第三級 | ||||||||||
| 資產: | ||||||||||||
| 現金等價物(貨幣市場基金) | $ | $ | $ | |||||||||
截至2023年12月31日,公司擁有
澳大利亞
稅收激勵 – IBAPL有資格從澳大利亞稅務局獲得現金退款,以補償符合條件的研究和開發
(「R&D」)支出,依據澳大利亞R&D稅收激勵計劃(「澳大利亞稅收激勵」)。
當相關支出已發生且金額可以可靠地衡量,並且能確認將收到澳大利亞稅收激勵時,澳大利亞稅收激勵將被視爲對R&D費用的減少。公司確認了額外的R&D費用爲$
已推遲
提供成本 — 公司已將與籌資工作相關的合格法律、會計和其他直接成本資本化
通過在7月自動櫃員機融資機制下出售其普通股來籌集資金。遞延發行成本已遞延並按比例攤銷
在7月自動櫃員機融資機制下出售後,作爲7月自動櫃員機收益的減少,將增加實收資本。由於該公司
暫停7月的自動櫃員機融資機制後,所有剩餘的延期發行成本立即攤銷爲額外的實收資本
減少了截至2024年9月30日的九個月中收到的收益。截至2024年9月30日,
基於股票的 補償 – 基於股票的補償費用代表公司股權獎勵的預計授予日期公平價值,包含在公司股票期權計劃下發放的股票期權和限制性普通股(見註釋7)。股權獎勵的公平價值在這些獎勵的必要服務期間內(通常是歸屬期)按直線法確認。公司在授予日使用布萊克-舒爾斯期權定價模型估算股票期權的公平價值,並在發生時確認作廢。對於歸屬取決於基於業績里程碑的股票獎勵,該費用在實現里程碑的可能性被認爲是很大的時點後的剩餘服務期間內確認,或者在業績控制項已經達到的情況下確認。
研究 和開發成本 – 研發費用在發生時計入費用。研發費用主要包括支付給顧問和外部服務提供商的臨床研究費用,以及與公司療法候選產品的設計、開發和測試有關的其他費用,以及與授權和里程碑成本相關的在許可證產品和科技方面的費用。獲得技術許可證所產生的費用如果該項已授權技術尚未達到商業可行性且沒有其他未來用途,則計入研發費用。公司購買的此類許可證需要在科研及開發、監管和市場批准工作方面達到實質性完成,以實現商業可行性並且沒有其他未來用途。
臨床 試驗成本是研發費用的一個組成部分。公司根據與臨床研究組織和實際臨床研究者簽訂的合同協議下所執行的服務,估算正在進行的臨床試驗所產生的費用。估算中包括(1)根據每個參與臨床試驗的機構與臨床試驗合同中規定的每位患者入組的費用,以及(2)從參與臨床試驗地點獲得的患者入組的逐步數據和實際執行的服務。臨床試驗假設的變化,例如估計入組所有患者的時間長度、篩查失敗率、患者脫落率、不良事件報告的數量和性質,以及入組的總患者數,都會影響每位患者的平均和預期成本以及臨床試驗的總體成本。公司監控試驗的進展及其相關活動,並在適用時調整費用計提。計提的調整在事實形成調整理由的時期內計入費用。
| 11 |
其他 綜合收益(損失) – 其他綜合收益(損失)包括外幣折算的收益和損失。折算收益和損失的累計金額在簡明合併資產負債表中作爲股東權益的一個單獨組成部分顯示,作爲累計其他綜合收益。
外幣翻譯與交易收益(損失) – 本公司及其全資子公司Nexcella使用美元維護會計記錄。本公司的運營子公司IBAPL位於澳洲,使用澳大利亞元維護其會計記錄,這是其功能貨幣。子公司的資產和負債根據資產負債表日的匯率轉換爲美元,股東權益帳戶按歷史匯率轉換,收入和費用使用該期間的平均匯率進行轉換。翻譯調整作爲其他綜合收益(損失)的單獨組成部分在合併運營和綜合損失報表中報告。以外幣計價的交易按交易日期近似有效匯率進行轉換。由外幣交易導致的收益(損失)包括在附帶的精簡合併操作和綜合損失報表中的一般和行政費用中,金額爲$(
資產 和設備 - 物業和設備中包括在建工程,涉及製造空間改進 幷包括施工、機械和設備的成本,以及在資產施工或安裝期間用於融資這些 資產的借款所產生的利息費用。在相關資產完成並準備好按預期使用之前,不對在建工程提取折舊。
公司資產的預計使用壽命如下:
| 使用壽命 | ||
| 操作設備 | ||
| 電子設備 | ||
| 辦公設備 |
售出或其他處置的資產的成本及相關累計折舊將從帳戶中消除,任何收益或損失將計入公司的營業結果。維護和修理費用在發生時確認爲費用;重要的更新和改善將資本化。
租賃 - 在合同開始時,公司確定該安排是否爲或包含租賃。經營租賃使用權(「ROU」)資產代表公司在租賃期限內使用基礎資產的權利,租賃負債代表公司因租賃產生的支付租賃款項的義務。經營租賃的ROU資產和負債在合同開始日期按租賃期限內租賃付款的現值進行確認。租賃費用在租賃期限內採用直線法確認。
公司已做出某些會計政策選擇,具體包括:\n(i)不對短期租賃(原始期限爲12個月或更短)確認使用權資產或租賃負債;(ii)將運營租賃的租賃和非租賃要素作爲單獨的租賃元件進行分離。截止到2024年9月30日和2023年12月31日,公司沒有任何融資租賃。
| 12 |
最近的 會計公告
在 2023年11月,財務會計準則委員會(「FASB」)發佈了 會計準則更新(「ASU」) 2023-07, 部門報告(主題280): 可報告部門披露的改進, 該準則要求每年和中期披露增量部門信息。此ASU自2023年12月15日之後開始的財年及自2024年12月15日之後開始的財年內的中期期間生效,採取追溯基礎。公司已於2024年1月1日實施此ASU,並確定不需要進行追溯性變更。
在 2023年12月,FASB 發佈了 ASU 2023-09, 所得稅(主題740): 對所得稅披露的改進, 該標準擴展了所得稅所需的披露。該ASU對2024年12月15日後開始的財政年度生效,允許提前採用。該修正案應在未來適用,同時允許追溯適用。公司目前正在評估該公告對其披露的影響。
注意 3 – 與Nexcella子公司的先前協議
Nexcella 吸收
在2024年5月20日,Nexcella與公司合併(「合併」),公司爲存續公司(「Nexcella吸收」)。合併根據特拉華州《一般公司法》(「DGCL」)第253條的規定進行,當公司向特拉華州國務卿提交了所有權和合並證書(「合併證書」)。在合併之前,公司持有Nexcella超過
創始人 協議
生效於 2022年12月8日,公司與Nexcella簽署了創始人協議(「Nexcella創始人協議」)。
Nexcella創始人協議規定,在合格首次公開募股(根據Nexcella修訂和重述的公司章程(「Nexcella COI」)的定義)或合格控制權變更(根據Nexcella COI的定義)之前,公司應根據Nexcella的要求,善意地向Nexcella提供資金,以高級無擔保的 promissory note 作爲證明。爲了爲Nexcella的設立和識別特定資產所花費的時間和資本,2022年12月21日,公司向Nexcella貸款約
| 13 |
每個
根據公司的選擇,A類優先股的股份可轉換爲Nexcella的一股已全額支付且不可評估的股份
普通股,但須進行某些調整。作爲NexcellaA類優先股的持有者,該公司每年三月收到
在所有已發行的A類優先股轉換爲Nexcella之日之前,13股(均爲 「PiK股息支付日期」)
普通股或已贖回(購買價格已全額支付),按比例分派的每股股息以額外已全額支付且不可估稅的形式支付
Nexcella普通股(「PiK股息」)的股份,即根據該標準發行的普通股總數
到這樣的 PiK 股息等於
此外,公司有權就其持有的每一股Nexcella普通股投票。除非法律或Nexcella公司組織章程另有規定,Nexcella A類普通股和A類優先股的持有者應與Nexcella普通股的持有者一起投票,作爲一個單一類別。
作爲對Nexcella創始人協議的額外考慮,Nexcella還同意:
| 14 |
管理 服務協議
有效
截至2022年12月8日,公司與Nexcella簽訂了管理服務協議(「Nexcella MSA」)。依照
根據Nexcella MSA的條款,該公司向Nexcella提供了管理、諮詢和諮詢服務。根據以下條件提供的服務
Nexcella MSA可能包括但不限於以下內容:(i) 有關Nexcella運營任何方面的建議和協助,
臨床試驗、財務規劃、戰略交易和融資,以及(ii)代表Nexcella與之建立關係
會計師、律師、財務顧問和其他專業人員(統稱爲 「服務」)。應委員會的要求
公司,Nexcella利用了臨床研究服務、醫學教育、傳播和營銷服務以及投資者關係/公共服務
公司指定的公司或個人的關係服務,前提是這些服務以市場價格提供。考慮中
對於服務,Nexcella向公司支付了每年的基本管理和諮詢費 $
Nexcella MSA於2024年5月20日因Nexcella吸收而終止。此外,由於Nexcella吸收,A類優先股、A類普通股和創始人協議不再存在。
註釋 4 – 預付款項及其他流動資產
預付費用 以及其他流動資產截至2024年9月30日和2023年12月31日的具體情況如下:
| 2024年9月30日 | 2023年12月31日 | |||||||
| 預付研發費用 | $ | $ | ||||||
| 預付保險費用 | ||||||||
| 預付投資者關係費用 | ||||||||
| 其他流動資產 | ||||||||
| 總預付費用和其他流動資產 | $ | $ | ||||||
注意 5 – 應付賬款及應計費用
應付賬款和預提費用截至2024年9月30日和2023年12月31日的組成如下:
| 2024年9月30日 | 2023年12月31日 | |||||||
| 應付賬款 | $ | $ | ||||||
| 已計提的研發費用 | ||||||||
| 應計專業服務費 | ||||||||
| 應計薪酬及相關費用 | ||||||||
| 其他應計費用 | ||||||||
| 應付賬款和應計費用總額 | $ | $ | ||||||
| 15 |
注意 6 – 物業及設備
截至2024年9月30日和2023年12月31日的財產和設備包括:
| 2024年9月30日 | 2023年12月31日 | |||||||
| 操作設備 | $ | $ | ||||||
| 辦公設備 | ||||||||
| 減:累計折舊 | ( | ) | ( | ) | ||||
| 建設中的工程 | ||||||||
| $ | $ | |||||||
截至2024年和2023年9月30日的九個月內,折舊費用總額爲$
正在進行的施工爲$
注意 7 – 股東權益
公司已授權 普通股股份和 優先股,每股面值爲$ 每股。
七月 2023年ATM銷售協議
在
2023年7月14日,公司與銷售代理簽訂了7月銷售協議,根據該協議,公司可以不時通過銷售代理提供和出售
公司的普通股(「7月股份」),面值$每股,須遵循7月銷售協議中所列的條款和條件。最初,公司有資格銷售高達
$
根據7月份銷售協議,銷售代理可以在被視爲「市場交易」的銷售中出售7月份的股份,這在《證券法》下的第415(a)(4)條規定中有定義,包括直接在納斯達克資本市場或公司普通股的任何其他現有交易市場上進行的銷售,在交易時以當時市場價格或與當時市場價格相關的價格進行的協商交易,及/或其他法律允許的任何方法。如果銷售無法按照公司不時指定的價格或以上價格進行,公司可以指示銷售代理不出售任何7月份的股份。
公司將向銷售代理支付固定佣金率爲
| 16 |
截至2024年9月30日的九個月期間,公司在七月ATM設施下共售出了 股普通股,合計淨收益爲$
普通 股票發行 – 公共發行
在
2024年2月5日,公司與Titan Partners Group LLC簽署了一份承銷協議(簡稱「協議」),該公司是美國資本合夥公司(American Capital Partners, LLC)的一個部門(簡稱「承銷商」),涉及一項承銷發行(簡稱「發行」)的 公司普通股的股份。公開發行價格爲每股$ 普通股,承銷商同意根據承銷協議以每股$ 的價格購買普通股。2024年2月8日,公司完成了發行,並在扣除承銷折扣和佣金以及估計發行費用後,收到了$
其他 普通股發行
在截至2024年9月30日的九個月期間,公司發行了 限售普通股股份,價值爲 $ 根據於2023年7月25日簽署的營銷服務協議,按過去10個交易日的平均收盤價計算,用於投資者關係服務。
在截至2024年9月30日的九個月期間,公司發行了 價值爲$的限制性普通股股份 根據營銷服務協議的延續,以收盤價格計算的投資者關係服務。
在截至2024年9月30日的九個月期間,公司發行了 在行使某些普通股期權時獲得普通股
現金收益爲$
截至2023年12月31日的年度內,公司簽署了多項營銷服務協議,依據該協議,公司同意發行 股普通股,總值爲$
限制性股票獎勵
根據合併, 公司向Nexcella 2022股權激勵計劃的前參與者發行, 限制性股票獎勵, 以接收公司普通股。這些股份按比例發行,導致公允價值沒有變化。
截至2024年9月30日的九個月期間,公司記錄了$的股票激勵費用 與之前發佈的限制性股票獎勵的總公允價值相關,這些費用包括在一般及管理費用中。未確認的股票激勵費用爲$ 與未歸屬的限制性普通股相關,預計將在剩餘的歸屬期間內確認 年。截止到2024年9月30日, 限制性普通股已歸屬,剩餘的 限制性股票將在歸屬期間內歸屬 年。
| 17 |
期權
在 2016年,公司的董事會批准了Immix Biopharma, Inc. 2016年股權激勵計劃(「2016計劃」)。 2016計劃允許董事會授予各種形式的激勵獎勵,涵蓋最多 普通股的 在截至2021年12月31日的年度內,董事會修訂了2016計劃,以增加2016計劃可發放的普通股總數量 至 普通股的。2021年9月10日,董事會批准了2021年 股權激勵計劃(經修訂和重述,簡稱「2021計劃」),根據該計劃初步保留併爲未來發放提供 (i) 普通股,外加(ii) 根據2016計劃保留但未發放的普通股數量,以及(iii) 根據2016計劃被註銷獎勵所對應的普通股數量,前提是 根據2021計劃與免稅獎勵(如2021計劃中定義)相關的普通股發放將不計入該股份限制。在2021年9月10日之後,2016計劃不再發放進一步的獎勵,但截至2021年9月10日的所有尚未過期的2016計劃獎勵(包括任何按祖父安排(如在2021計劃中定義)) 仍將繼續受2016計劃及任何適用獎勵協議中規定的條款、條件和程序的約束。
2023年4月24日,公司的董事會通過了Immix Biopharma, Inc.修訂和重述的2021年綜合股權激勵計劃(「修訂的2021年計劃」),該計劃在其他事項中,將可以根據該計劃發行的普通股數量增加了 股,需經股東批准。2023年6月7日,公司的股東批准了修訂的2021年計劃。2024年4月18日,我們的董事會批准了2021年計劃的修訂(「修訂的2021年計劃」)nd (i) 將2021年計劃下可發行普通股的數量增加到 一個總股份儲備 以及(ii)採納2021年計劃的常青條款,以在未來十年內爲2021年計劃下可發行普通股的自動年度增加提供支持(「2021年計劃修訂」)。根據常青條款,在接下來的十年內,2021年計劃下可發行股份的數量將自動在每年的1月1日增加,開始於2025年1月1日,結束於(幷包括)2034年1月1日,增加的數量等於截至前一個日曆年12月31日的普通股總數的五個百分點(%)在2024年6月11日,公司股東批准了修訂的nd 修訂後的2021計劃。截至2024年9月30日, 還有 公司普通股可根據修訂後的2021計劃發行。
此外,公司向Nexcella 2022年股權激勵計劃的前參與者發行了購買高達的期權, 公司普通股的每股行權價爲$ (2024年5月17日的收盤價),依據公司的2021年修訂及重述的綜合股權激勵計劃。期權是按比例發行的,並沒有導致公平價值的變化。
在截至2024年9月30日的九個月期間,董事會的薪酬委員會批准發行期權用於購買 公司的普通股給公司的非員工董事會成員,以及 公司的普通股給公司的管理層。期權的有效期限爲 年,行使價格爲$ 每股, .
截至2024年9月30日的九個月期間,董事會批准向公司員工發行購買期權 公司的普通股,期限爲 年,行使價格範圍從 $ - $ 每股,這些期權.
公司確認了與期權相關的股票薪酬金額爲$ 和 $ 截止到2024年和2023年9月30日的三個月內與期權相關的金額爲$ 和 $ 截止到2024年和2023年9月30日的九個月內與期權相關的金額爲$,該支出包含在一般和管理費用中。
截至2024年9月30日,公司尚未確認的股票薪酬費用爲$與尚未歸屬的期權相關, 預計將在加權平均歸屬期內確認 年。
| 18 |
| 選項 | 加權- 平均行使 每股價格 | |||||||
| 傑出,2024年1月1日 | $ | |||||||
| 授予 | $ | |||||||
| 已行使 | ( | ) | $ | |||||
| 被註銷 | ( | ) | $ | |||||
| 已到期 | $ | |||||||
| 未兌現且預計將於2024年9月30日兌現 | $ | |||||||
| 未償還的 | 可行使 | |||||||||||||||||||||
| 行權價格範圍 | 數量 期權 股份 | 加權 平均 行使價格 | 加權 平均 剩餘 生命(年) | 數量 期權 股份 | 加權 平均 行使價格 | |||||||||||||||||
| $ | - | $ | $ | |||||||||||||||||||
| $ | - | $ | $ | |||||||||||||||||||
| $ | - | $ | $ | |||||||||||||||||||
| $ | - | $ | $ | |||||||||||||||||||
| $ | $ | |||||||||||||||||||||
累積內在價值是通過計算基礎股票期權的行使價格與公司普通股的公允價值之間的差額得出的,對於在期末處於價內的股票期權。截止到2024年9月30日,已歸屬和未行使的期權的累積內在價值爲$.
截至2024年9月30日的九個月期間,行使的股票期權的總內在價值爲$.
股票 認股權證
以下表格總結了截至2024年9月30日的九個月的股票權證活動:
| 認購權證 | 加權平均 每股行使價格 分享 | |||||||
| 截至2024年1月1日,未到期且可行使 | $ | |||||||
| 授予 | $ | |||||||
| 已行使 | $ | |||||||
| 被註銷 | $ | |||||||
| 已到期 | $ | |||||||
| 未行使且可行使,2024年9月30日 | $ | |||||||
| 19 |
| 未償還的 | 可行使 | |||||||||||||||||||||
| 行使價格 | 數量 Warrants 股份 | 加權 平均 行使價格 | 加權 平均 剩餘 生命(年) | 數量 Warrants 股份 | 加權 平均 行使價格 | |||||||||||||||||
| $ | $ | - | $ | |||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||||
| $ | $ | $ | ||||||||||||||||||||
| $ | $ | |||||||||||||||||||||
合併 內在價值是計算標的股票認股權證的行使價格與公司普通股的公允價值之間的差額,對於在期末時已在價內的股票認股權證。截止到2024年9月30日,已歸屬和未行使的認股權證的內在價值爲$.
Nexcella 股權交易
Nexcella 2022年股權激勵計劃(「2022計劃」)允許Nexcella的董事會授予各種形式的激勵獎勵,最初覆蓋多達 股份普通股。2023年5月29日,Nexcella的董事會批准了第二次修訂和重新制定的Nexcella 2022年股權激勵計劃,增加了可根據該計劃發行的Nexcella普通股的數量,從 股份數量爲 股份。2023年8月11日,Nexcella的董事會請求第三次修訂和重新制定的2022年股權激勵計劃,增加了可根據該計劃發行的Nexcella普通股的數量,從 至 股份。Nexcella的股東隨後批准了根據該計劃發行的Nexcella普通股的數量增加到 股份。2024年5月17日,在併入公司後,2022計劃宣佈停止存在。
普通 股票
根據《創始人協議》的條款,Nexcella在2024年3月13日向公司發行了 基於截至2024年3月12日Nexcella已發行的總稀釋股份,作爲PIK分紅髮放的普通股。
限制性股票獎勵
在截至2024年9月30日的三個月和九個月期間,公司記錄了股票獎勵補償費用$ 和 $,分別, 這與之前發行的限制性股票獎勵的總公允價值有關,該公允價值已計入一般和管理費用。 根據合併的規定,公司向Nexcella 2022年股權激勵計劃的前參與者發行了 限制性 股票獎勵,以接收公司普通股。這些股份是按比例發行的,因此公允價值沒有變化。 因此,Nexcella沒有剩餘的未歸屬股票補償費用。
期權
公司確認了與期權相關的股票薪酬金額爲$ 和 $ 與截至2024年9月30日的三個月和九個月的期權相關,包含在一般和管理費用中。根據合併的規定,公司向Nexcella 2022年股權激勵計劃的前參與者發放了購買最多的期權, 根據公司修訂的2021年全方位股權激勵計劃發放了公司普通股的股份。這些期權按比例發放,未導致公允價值的變化。因此,Nexcella下沒有剩餘的未歸屬的基於股票的補償費用。
| 20 |
| 選項 | 加權- 平均行使 每股價格 | |||||||
| 截至2024年1月1日,未到期且可行使 | $ | |||||||
| 授予 | $ | |||||||
| 已行使 | $ | |||||||
| 被註銷 | ( | ) | $ | |||||
| 已到期 | $ | |||||||
| 未兌現且預計將於2024年9月30日兌現 | $ | |||||||
Note 8 – Licenses Acquired
Research and License Agreement with HADASIT and BIRAD
On
December 8, 2022, Nexcella entered into a Research and License agreement with HADASIT and BIRAD (collectively, the “Licensors”)
to acquire intellectual property rights pertaining to CAR-T (the “H&B License”). Pursuant to the H&B License, Nexcella
paid the Licensors an upfront license fee of $
During
the nine months ended September 30, 2024 and 2023, the Company recorded R&D expenses of $
Patent License Agreement with U.S. Medical Research Foundation
在2024年8月,公司與一家美國醫療研究基金會簽署了一項專利許可協議(「許可協議」),根據該協議,公司獲得了某些獨佔和非獨佔的許可及轉許可,以用於電芯治療產品(「授權產品」)的開發和商業化。根據許可協議的條款,公司應分三期支付總額爲$的預付款。
| 21 |
注意 9 - CIRm 贈款
在2024年7月25日,公司獲得了來自加利福尼亞再生醫學研究所的一筆百萬美元的補助金,
注意 10 – 租賃
在
2024年1月,公司簽署了一項長期經營租賃協議,
截至2024年9月30日的三個月和九個月的運營租賃成本元件,記錄在隨附的簡明合併運營報表中的一般和行政費用如下:
截至三個月 2024年9月30日 | 截至九個月 2024年9月30日 | |||||||
| 運營租賃成本 | $ | $ | ||||||
| 短期租賃成本 | ||||||||
| 總租賃成本 | $ | $ | ||||||
下表總結了截至2024年9月30日在合併資產負債表中記錄的與租賃相關的資產和負債:
| 2024年9月30日 | ||||
| 經營租賃 | - | |||
| 經營租賃使用權資產 | $ | |||
| 使用權負債經營租賃的當前部分 | $ | |||
| 使用權負債經營租賃的長期部分 | ||||
| 總經營租賃負債 | $ | |||
公司在確定租賃支付的現值時使用增量借款利率,除非隱含利率易於確定。公司估計其增量借款利率爲
以下表格提供了截至2024年9月30日的租賃負債到期情況:
| 事件 | ||||
| 租賃 | ||||
| 2024年(剩餘3個月) | $ | |||
| 2025 | ||||
| 2026 | ||||
| 2027 | ||||
| 2028年及之後 | ||||
| 未來未折現的租賃支付總額 | ||||
| 減:利息 | ( | ) | ||
| 租賃負債的現值 | $ | |||
| 22 |
Note 11 – Commitments and Contingencies
Indemnifications
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and may provide for indemnification of the counterparty. The Company’s exposure under these agreements is unknown because it involves claims that may be made against it in the future, but have not yet been made. To date, the Company has not been subject to any claims or been required to defend any action related to its indemnification obligations.
The Company indemnifies each of its directors and officers for certain events or occurrences, subject to certain limits, while the director is or was serving at the Company’s request in such capacity, as permitted under Delaware law and in accordance with its certificate of incorporation and bylaws. The term of the indemnification period lasts as long as the director or officer may be subject to any proceeding arising out of acts or omissions of such individual in such capacity. The maximum amount of potential future indemnification is unlimited. The Company believes that the fair value of these indemnification obligations is minimal. Accordingly, the Company has not recognized any liabilities relating to these obligations as of September 30, 2024.
Legal Proceedings
From time to time the Company may be involved in claims that arise during the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, the Company does not currently have any pending litigation to which it is a party or to which its property is subject that it believes to be material. Regardless of the outcome, litigation can be costly and time consuming, and it can divert management’s attention from important business matters and initiatives, negatively impacting the Company’s overall operations.
Employment Agreements
On
June 18, 2021, the Company entered into an Employment Agreement with Ilya Rachman (as amended, the “Rachman Employment Agreement”),
effective for a three-year term, subject to the terms of the agreement which provide that unless the Company and Dr. Rachman have otherwise
agreed in writing, if Dr. Rachman continues to work for the Company after the expiration of the term (which he has), his employment shall
be under the same terms and conditions provided for in the Rachman Employment Agreement, except that his employment will be on an “at
will” basis and the provisions of the agreement allowing for Dr. Rachman to terminate the agreement for “good reason”
and for Dr. Rachman to be paid severance in the event his employment is terminated by the Company without cause or by Dr. Rachman for
good reason will no longer apply, and the Rachman Employment Agreement currently remains in effect pursuant to such terms. Pursuant to
the Rachman Employment Agreement, the Company employs Dr. Rachman as Chief Executive Officer and Dr. Rachman was entitled to a base salary
of $
| 23 |
On
March 18, 2021, the Company entered into a Management Services Agreement with Alwaysraise LLC, an entity which Gabriel Morris, the Company’s
Chief Financial Officer and a member of the Board, is sole member, which was amended effective June 18, 2021 (as amended, the “Morris
MSA”). The Morris MSA had an initial two-year term, automatically renewable thereafter for successive one year terms unless terminated
by either party, and currently has a term through March 18, 2025. Pursuant to the Morris MSA, the Company employs Mr. Morris as Chief
Financial Officer and Mr. Morris was entitled to a base salary of $
On June 24, 2021, the Company issued an offer letter to Graham Ross Oncology Consulting Services Ltd., a United Kingdom company, of which Graham Ross, the Company’s Acting Chief Medical Officer and Head of Clinical Development is the sole member, regarding Dr. Ross’s provision of consultative services to the Company (the “Offer Letter”). Pursuant to the Offer Letter (signed by Dr. Ross on June 24, 2021), Dr. Ross is entitled to an hourly rate for his consulting services and an option grant. On June 24, 2021, the Company also signed a mutual confidentiality and non-disclosure agreement with Graham Ross Oncology Consulting Services Ltd.
Collaboration Agreement
In August 2021, the Company entered into a Clinical Collaboration and Supply Agreement with BeiGene Ltd. (“BeiGene”) for a combination Phase 1b clinical trial in solid tumors of IMX-110 and anti-PD-1 Tislelizumab (the subject of a collaboration and license agreement among BeiGene and Novartis). Under the terms of the agreement, the Company will conduct the combination trial. The cost of Tislelizumab manufacture and supply (including shipping, taxes and duty if applicable and any third-party license payments that may be due) will be solely borne by BeiGene. To date, no amounts have been paid to BeiGene.
Note 12 – Subsequent Events
Common Stock Issuance – Marketing Services Agreements
Subsequent
to September 30, 2024, the Company issued shares of restricted common stock valued at $
| 24 |
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You should read the following discussion and analysis of our financial condition and results of operations together with our unaudited interim condensed consolidated financial statements and the related notes appearing elsewhere in this Quarterly Report on Form 10-Q. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, as may be amended, supplemented or superseded from time to time by other reports we file with the SEC. All amounts in this report are in U.S. dollars, unless otherwise noted.
Throughout this Quarterly Report on Form 10-Q, references to “we,” “our,” “us,” the “Company,” “Immix,” or “Immix Biopharma” refer to Immix Biopharma, Inc., individually, or as the context requires, collectively with its subsidiaries.
Our logo and some of our trademarks and tradenames are used in this Report. This Report also includes trademarks, tradenames and service marks that are the property of others. Solely for convenience, trademarks, tradenames and service marks referred to in this Report may appear without the ®, ™ and SM symbols. References to our trademarks, tradenames and service marks are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensors if any, nor that respective owners to other intellectual property rights will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend the use or display of other companies’ trademarks and trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
Certain capitalized terms used below and otherwise defined below, have the meanings given to such terms in the footnotes to our unaudited consolidated financial statements included above under “Part I – Financial Information” – “Item 1. Financial Statements”.
Unless the context otherwise requires and for the purposes of this Report only:
● “Exchange Act” refers to the Securities Exchange Act of 1934, as amended;
● “SEC” or the “Commission” refers to the United States Securities and Exchange Commission; and
● “Securities Act” refers to the Securities Act of 1933, as amended.
Available Information
We file annual, quarterly, and current reports, proxy statements and other information with the Securities and Exchange Commission. Our SEC filings (reports, proxy information statements, and other information) are available to the public over the Internet at the SEC’s website at www.sec.gov and are available for download, free of charge, soon after such reports are filed with or furnished to the SEC, on the “Investor & News,” “SEC Filings” page of our website at www.immixbio.com. Copies of documents filed by us with the SEC are also available from us without charge, upon oral or written request to our Secretary, who can be contacted at the address and telephone number set forth on the cover page of this Report. The information contained on the websites referenced in this Report is not incorporated by reference into this filing. Further, the Company’s references to website URLs are intended to be inactive textual references only.
Overview
Immix Biopharma, Inc. is a clinical-stage biopharmaceutical company focused on the application of chimeric antigen receptor cell therapy (“CAR-T”) in light chain (AL) Amyloidosis and select immune-mediated diseases. Our lead cell therapy candidate is U.S. Food and Drug Administration (“FDA”) investigational new drug (“IND”) cleared CAR-T NXC-201, currently being evaluated in our ongoing United States Phase 1b/2 NEXICART-2 (NCT06097832) clinical trial and our ex-U.S. phase 1b/2a NEXICART-1 (NCT04720313) clinical trial.
NXC-201 has been awarded Orphan Drug Designation (“ODD”) by both the FDA and European Commission (“EMA”) in AL Amyloidosis.
| 25 |
Our mission is to harness the immune system through innovative cell therapies and other modalities to deliver widely accessible cures in select immune-mediated diseases and other indications, as we believe patients are waiting.
Our strategy is to:
| ● | Develop our lead candidate CAR-T NXC-201 in AL Amyloidosis and select immune-mediated diseases; and | |
| ● | Pursue development of NXC-201 and additional cell therapy candidates in other applicable indications where CAR-T is not an approved therapy today. |
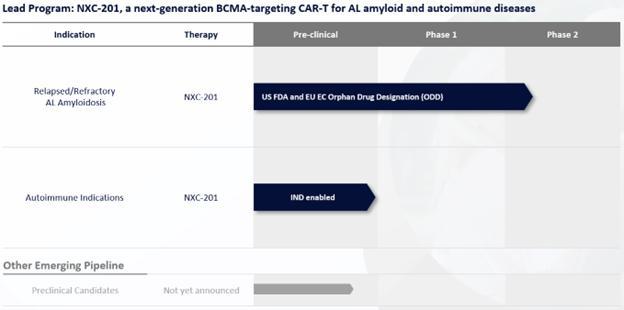
Our N-GENIUS platform has produced our clinical-stage lead candidate NXC-201, a next-generation CAR-T for AL Amyloidosis and select immune-mediated diseases.
Figure 1: ImmixBio Pipeline

NXC-201 is in clinical trials to treat relapsed/refractory AL Amyloidosis.
AL amyloidosis is a life-threatening immunological disorder in which an abnormal protein called amyloid builds up in tissues and organs. This abnormal protein is produced by long-lived plasma cells (“LLPCs”), a type of immune B-cell. The signs and symptoms of AL amyloidosis vary among patients because build-up may occur in the heart (most frequent cause of mortality), liver, kidneys, intestines, muscles, joints, nerves, or spleen, according to the National Institutes of Health (“NIH”). Diagnosis is frequently delayed, due to varied and non-specific symptoms including: fatigue, weight loss, shortness of breath, dizziness, and numbness in hands and feet. Upon diagnosis, many patients already have late-stage disease, and are not aware of available treatment options and clinical trials.
As of September 2024, there are no FDA approved drugs for relapsed/refractory AL Amyloidosis.
The U.S. observed prevalence of relapsed/refractory AL Amyloidosis is growing 12% per year according to Staron, et al Blood Cancer Journal 2021, estimated to reach 33,277 patients in 2024. Untreated patients with AL amyloidosis and cardiac involvement have a median survival of less than 1 year, according to Quock, et al. Journal of Comparative Effective Research, 2023. The current market size for amyloidosis therapies is estimated at $3.6 billion, expected to reach $6 billion in 2027, according to Grand View Research.
| 26 |
As of September 2024, we have treated 3 relapsed/refractory AL Amyloidosis patients in the United States in our ongoing Phase 1b/2 multi-site NEXICART-2 (NCT06097832) U.S. clinical trial. Memorial Sloan Kettering Cancer Center is the lead NEXICART-2 clinical site.
As of September 30, 2024, we have treated 13 relapsed/refractory AL Amyloidosis patients in our ongoing Phase 1b/2a NEXICART-1 (NCT04720313) ex-U.S. clinical trial.
In September 2023, the FDA granted ODD to NXC-201 for the treatment of AL Amyloidosis. If a product that has ODD subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusive approval (or exclusivity), which means that the FDA may not approve any other applications to market the same drug for the same indication for 7 years (except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity).
In November 2023, the FDA cleared an IND application for NXC-201 to enroll U.S. patients into NXC-201 clinical trials.
In December 2023, NXC-201 clinical data in relapsed/refractory AL Amyloidosis was presented in an oral presentation at the 65th annual American Society of Hematology (“ASH”) meeting, covering 10 relapsed/refractory AL Amyloidosis patients treated with NXC-201, indicating an overall response rate of 100% (10/10) and a complete response rate of 70% (7/10).
In February 2024, the European Commission (“EC”) granted orphan drug designation to NXC-201 for the treatment of AL Amyloidosis. Benefits of European ODD include: 10 years of market exclusivity once authorized in the EU; Access to the EU centralized authorization procedure; and reduced fees for EU protocol assistance, marketing authorization applications, inspections before authorization, applications for changes to marketing authorizations made after approval, and reduced annual fees.
Our Other Programs
Our other programs include NXC-201 for select immune-mediated diseases, a $25 billion combined annual market size according to Grand View Research and Fortune Business Insights and other preclinical candidates.
While our focus is NXC-201 in AL Amyloidosis and select immune-mediated diseases, we continue to collect and organize IMX-110 data in monotherapy for soft tissue sarcoma, a $3 billion market size according to Medgadget, and in combination with anti-PD-1 for colorectal cancer, a $27 billion market size according to IndustryARC, to evaluate next steps.
Since inception, we have devoted substantially all of our resources to developing product and technology rights, conducting research and development, organizing and staffing our Company, business planning and raising capital. We operate as one business segment and have incurred recurring losses, the majority of which are attributable to research and development activities and negative cash flows from operations. We have funded our operations primarily through the sale of equity securities. Currently, our primary use of cash is to fund operating expenses, which consist primarily of research and development expenditures, and to a lesser extent, general and administrative expenditures. We expect to continue to incur significant expenses and operating losses for the foreseeable future as we advance our product candidates through all stages of development and clinical trials and, ultimately, seek regulatory approval. In addition, if we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. Furthermore, we incur costs associated with operating as a public company, including significant legal, accounting, investor relations and other expenses. Our net losses may fluctuate significantly from quarter-to-quarter and year-to-year, depending on the timing of our clinical trials and our expenses on other research and development activities.
| 27 |
Absorption of Nexcella Subsidiary
On May 20, 2024, Nexcella, was merged (the “Merger”) with and into the Company, with the Company as the surviving corporation. The Merger was effected pursuant to Section 253 of the Delaware General Corporation Law (“DGCL”) when the Company filed a Certificate of Ownership and Merger (“Certificate of Merger”) with the Secretary of State of the State of Delaware. Immediately prior to the Merger, the Company owned greater than 95% of the outstanding common stock on a fully diluted basis of Nexcella, par value $0.0001 per share (the “Nexcella Shares”), and 100% of the outstanding shares of each other class of capital stock of Nexcella. Under the DGCL, the only approval required was that of the Company’s Board of Directors for the Merger to become effective. As a result of the Merger, Nexcella ceased to exist and all assets, operations and other property and rights of Nexcella have been succeeded to by the Company. Pursuant to the terms of the Certificate of Merger, as a result of the Merger, each of the outstanding Nexcella Shares (other than Nexcella Shares held by the Company) were converted, into common stock of the Company (“Company Merger Shares”). In connection with the Merger, the Company issued 989,876 shares of its common stock to the former stockholders of Nexcella (other than shares held by the Company) (including Company common stock issued to third-party cash investors in Nexcella) (the “Merger Shares”). In addition, the Company issued to the former participants in the Nexcella 2022 Equity Incentive Plan, 275,759 restricted stock awards to receive common stock in the Company and options to purchase up to 595,676 shares of Company common stock at an exercise price of $2.47 per share (the closing price on May 17, 2024), under the Company’s Amended and Restated 2021 Omnibus Equity Incentive Plan.
Research and License Agreement with Hadasit and BIRAD
On December 8, 2022, our subsidiary Nexcella entered into a Research and License Agreement (the “Agreement”) with Hadasit Medical Research Services & Development, Ltd. and BIRAD – Research and Development Company Ltd. (collectively, the “Licensors”) pursuant to which the Licensors granted to Nexcella an exclusive, worldwide, royalty-bearing license throughout the world, except Israel, Cyprus and other countries in the Middle East (the “Territory”) to an invention entitled “Anti-BCMA CAR-T cells to target plasma cell” to develop, manufacture, have manufactured, use, market, offer for sale, sell, have sold, export and import Licensed Product (as defined in the Agreement). Pursuant to the Agreement, Nexcella paid the Licensors an upfront fee of $1,500,000 in December 2022. Additional quarterly payments totaling approximately $13.0 million are due through September 2026 along with an annual license fee of $50,000. Nexcella has agreed to pay royalties to the Licensors equal to 5% of Net Sales (as defined in the Agreement) during the Royalty Period. “Royalty Period” means for each Licensed Product, on a country-to-country basis, the period commencing on December 8, 2022, and ending on the later of (a) the expiration of the last to expire Valid Claim (as defined in the Agreement) under a Licensed Patent (as defined in the Agreement), if any, in such country, (b) the date of expiration of any other Exclusivity Right (as defined in the Agreement) or data protection period granted by a regulatory or other governmental authority with respect to a Licensed Product, or (c) 15 years from the date of First Commercial Sale (as defined in the Agreement) of a Licensed Product in such country.
In addition, Nexcella is required to pay sales milestone payments of up to $20 million for Net Sales exceeding $700 million and Nexcella has committed to funding NXC-201 clinical trials in Israel over four years for an estimated total cost of approximately $13 million, spread out on a quarterly basis over that period, which Nexcella believes will generate clinical trial data owned by Nexcella. The term of the Agreement commenced on December 8, 2022 and, unless earlier terminated pursuant to the terms thereof, will continue in full force and effect until the later of the expiration of the last Valid Claim under a Licensed Patent or a Joint Patent (as defined in the Agreement) or Exclusivity Right covering a Licensed Product or the expiration of a continuous period of 15 years during which there shall not have been a First Commercial Sale of any Licensed Product in any country in the world. Licensors may terminate the Agreement immediately if Nexcella or its affiliates or sublicensees commences an action in which it challenges the validity, enforceability or scope of any of the Licensed Patents or Joint Patents. In addition, either party may terminate the Agreement if the other party materially breaches the Agreement and fails to cure such breach within 30 days. Additionally, Licensors may terminate the Agreement if Nexcella becomes insolvent or files for bankruptcy.
The license remains with the Company after the Nexcella Absorption.
| 28 |
July 2023 ATM Offering
On July 14, 2023, we entered into an ATM Sales Agreement (the “July Sales Agreement”) with the Sales Agent pursuant to which we may offer and sell, from time to time, through the Sales Agent, shares of our common stock, subject to the terms and conditions set forth in the July Sales Agreement. Initially, we are eligible to sell up to $4,200,000 worth of shares of our common stock as the aggregate market value of our shares of common stock eligible for sale under the July Sales Agreement is subject to the limitations of General Instruction I.B.6 of Form S-3 until such time that our public float equals or exceeds $75.0 million. In the event the aggregate market value of our outstanding common stock held by non-affiliates equals or exceeds $75.0 million, then the one-third limitation on sales set forth in General Instruction I.B.6 of Form S-3 will not apply to additional sales made pursuant to the July Sales Agreement. We agreed to pay the Sales Agent a commission rate of 3.75% of the aggregate gross proceeds from the sale of the shares of our common stock pursuant to the July Sales Agreement and have paid an expense deposit of $15,000 to the Sales Agent, which will be applied against the actual out-of-pocket accountable expenses. In addition, we have agreed to reimburse the Sales Agent for all expenses related to the offering including, without limitation, the fees and expenses of the Sales Agent’s legal counsel up to $50,000, and to reimburse the Sales Agent, upon request, for such costs, fees and expenses in an amount not to exceed $7,500 on a quarterly basis for the first three fiscal quarters of each year and $10,000 for the fiscal fourth quarter of each year. The offering pursuant to the July Sales Agreement will terminate upon the earlier of (i) the sale of all of the shares of common stock subject to the July Sales Agreement and (ii) termination of the July Sales Agreement as permitted therein. We may terminate the July Sales Agreement in our sole discretion at any time by giving ten days’ prior notice to the Sales Agent. The Sales Agent may terminate the July Sales Agreement under the circumstances specified in the July Sales Agreement and in its sole discretion at any time by giving ten days’ prior notice to us. In addition, the July Sales Agreement may be terminated upon mutual agreement by us and the Sales Agent.
From July 14, 2023 through February 5, 2024, the Company has sold 328,136 common shares pursuant to the July ATM Facility for net proceeds of $1,091,887, after offering expenses. On February 5, 2024, the Company suspended, and is not offering any shares of its common stock pursuant to, the prospectus supplement dated July 14, 2023, relating to the July Sales Agreement by and between the Company and ThinkEquity LLC. The Company will not make any sales of common stock pursuant to the July Sales Agreement unless and until a new prospectus supplement is filed with the SEC; however, the Sales Agreement remains in full force and effect.
Public Offering
On February 5, 2024, the Company entered into an Underwriting Agreement (the “Agreement”) with Titan Partners Group LLC, a division of American Capital Partners, LLC (the “Underwriter”), relating to an underwritten offering (the “Offering”) of 5,535,055 shares of common stock of the Company. The public offering price was $2.71 per share of Common Stock and the Underwriter agreed to purchase the Common Stock pursuant to the Underwriting Agreement at a price of $2.5203 per share. On February 8, 2024, the Company closed the offering and received net proceeds of $13,565,760, after deducting underwriting discounts and commissions and estimated offering expenses. Pursuant to the Agreement, the Company granted the Underwriter a 30-day over-allotment option to purchase up to an additional 783,970 shares of Common Stock which was exercised in full on March 1, 2024 for net proceeds of $1,954,594, after deducting underwriting discounts and offering expenses.
Results of Operations
Three Months Ended September 30, 2024 compared to the Three Months Ended September 30, 2023
General and Administrative Expense
General and administrative expenses were $2,949,403 for the three months ended September 30, 2024, compared to $2,417,776 for the three months ended September 30, 2023.
The expenses incurred in both periods were related to salaries, patent maintenance costs and general accounting and other general consulting expenses, which were higher for the three months ended September 30, 2024, due to increased investor relations services of $543,383, increased compensation expense of $355,670, due to hiring of additional employees; and increased other general expenses of $11,227, offset by decreased professional services of $309,561 primarily driven by a significant decrease in legal fees related to the Nexcella subsidiary and decreased stock-based compensation of $69,092 from a reduction in equity awards issued.
Research and Development Expense
Research and development expense was $4,445,528 for the three months ended September 30, 2024, compared to $2,106,020 for the three months ended September 30, 2023.
The increased research and development expenses during the three months ended September 30, 2024, as compared to the three months ended September 30, 2023, were related to our ongoing Phase 1b/2a clinical trial and our CAR-T clinical trial, including, but not limited to, CRO and related costs for maintaining and treating patients in the clinical trial, as well as site onboarding costs and license fees.
| 29 |
Interest Income
Interest income was $256,680 for the three months ended September 30, 2024, compared to $186,691 for the three months ended September 30, 2023. Interest income in the current period was related to interest received on investments in a money market fund and increased from the prior period as a result of the Company maintaining higher balances in money market funds during the current period.
Provision for Income Taxes
Provision for income taxes for the three months ended September 30, 2024 was $11,144, compared to $6,807 for the three months ended September 30, 2023, due to withholding taxes relating to our Australian subsidiary.
Net Loss
Net loss for the three months ended September 30, 2024 was $7,149,395, compared to $4,343,912 for the three months ended September 30, 2023, which increase was due primarily to the increase in general and administrative expenses and research and development expenses, as discussed in greater detail above.
Nine Months Ended September 30, 2024 compared to the Nine Months Ended September 30, 2023
General and Administrative Expense
General and administrative expenses were $7,769,224 for the nine months ended September 30, 2024, compared to $5,130,977 for the nine months ended September 30, 2023.
The expenses incurred in both periods were related to salaries, patent maintenance costs and general accounting and other general consulting expenses, which were higher for the nine months ended September 30, 2024, due to increased investor relations services of $1,199,230, increased professional services of $65,764, both due to service scope expansion and price increases, increased compensation of $548,112 due to hiring additional employees, increased stock-based compensation of $462,645 from additional equity awards issued, and increased other general expenses of $362,496.
Research and Development Expense
Research and development expense was $9,918,336 for the nine months ended September 30, 2024, compared to $5,634,284 for the nine months ended September 30, 2023.
The increased research and development expenses during the nine months ended September 30, 2024, as compared to the nine months ended September 30, 2023, were related to our ongoing Phase 1b/2a clinical trial and our CAR-T clinical trial, including, but not limited to, CRO and related costs for maintaining and treating patients in the clinical trial, as well as site onboarding costs and license fees.
Interest Income
Interest income was $831,503 for the nine months ended September 30, 2024, compared to $343,431 for the nine months ended September 30, 2023. Interest income in the current period was related to interest received on investments in a money market fund, which increased as a result of the Company maintaining higher balances in money market funds during the current period.
Provision for Income Taxes
Provision for income taxes for the nine months ended September 30, 2024 was $30,252 compared to $18,326 for the nine months ended September 30, 2023, due to withholding taxes relating to our Australian subsidiary.
| 30 |
Net Loss
Net loss for the nine months ended September 30, 2024 was $16,886,309 compared to $10,440,156 for the nine months ended September 30, 2023, which increase was due primarily to the increase in general and administrative expenses and research and development expenses, each as discussed in greater detail above.
Liquidity and Capital Resources
Our primary use of cash is to fund operating expenses, which consist of research and development expenditures and various general and administrative expenses. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable, accrued expenses and prepaid expenses.
Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| ● | the scope, timing, progress and results of discovery, pre-clinical development, laboratory testing and clinical trials for our product candidates; | |
| ● | the costs of manufacturing our product candidates for clinical trials and in preparation for regulatory approval and commercialization; | |
| ● | the extent to which we enter into collaborations or other arrangements with additional third parties in order to further develop our product candidates; | |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; | |
| ● | the costs and fees associated with the discovery, acquisition or in-license of additional product candidates or technologies; | |
| ● | expenses needed to attract and retain skilled personnel; | |
| ● | the costs associated with being a public company; | |
| ● | the costs required to scale up our clinical, regulatory and manufacturing capabilities; | |
| ● | the costs of future commercialization activities, if any, including establishing sales, marketing, manufacturing and distribution capabilities, for any of our product candidates for which we receive regulatory approval; and | |
| ● | revenue, if any, received from commercial sales of our product candidates, should any of our product candidates receive regulatory approval. |
As of September 30, 2024, we had $16.2 million of working capital.
On July 25, 2024, the Company was awarded an $8 million grant from the California Institute for Regenerative Medicine (CIRM) to support the clinical development of chimeric antigen receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of 2024.
| 31 |
We believe that our existing cash and cash equivalents as of September 30, 2024 will enable us to fund our operating expenses and capital expenditure requirements for at least the next 12 months. However, we may need additional funds depending on our operational needs and capital requirements for clinical trials, other research and development expenditures, and general and administrative expenses. We currently have no credit facility or committed sources of capital.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our operations through a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution arrangements, other collaborations, strategic alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, which dilution may be significant, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our research, product development or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Cash used in operating activities
Net cash used in operating activities was $13,118,904 for the nine months ended September 30, 2024 and $8,694,001 for the nine months ended September 30, 2023. Net cash used in operating activities for the nine months ended September 30, 2024 was primarily related to our net loss of $16,886,309, offset by non-cash items of stock-based compensation expense of $2,314,920, depreciation expense of $12,338 and right of use asset amortization of $61,713. Operating activities also included an increase in accounts payable and accrued expenses of $2,120,531, an increase in the tax receivable of $746,748, and an increase in prepaid expenses of $7,932. Net cash used in operating activities for the nine months ended September 30, 2023, was primarily related to our net loss of $10,440,156, offset by non-cash items of stock-based compensation expense of $1,514,900 and depreciation expense of $2,392. Operating activities also included an increase in accounts payable of $1,580,248, an increase in the tax receivable of $427,476 and an increase in prepaid expenses of $923,909.
Cash used in investing activities
Net cash used in investing activities was $670,529 for the nine months ended September 30, 2024, consisting solely of purchase of property and operating equipment, compared to $38,912 for the nine months ended September 30, 2023.
Cash provided by financing activities
Net cash provided by financing activities was $15,948,567 for the nine months ended September 30, 2024 and $14,876,820 for the nine months ended September 30, 2023. Net cash provided by financing activities in 2024 was related to proceeds of $15,946,078 from the sale of common shares through a public offering. Net cash provided by financing activities in 2023 was related to proceeds of $5,002,284 from the sale of common shares through the at-the-market offerings, proceeds of $9,934,153 from the sale of common shares and pre-funded warrants in a private placement offering, and proceeds of $175,000 from the sale of common shares of our former majority-owned (now wholly-owned) subsidiary, Nexcella, offset by payments of deferred offering costs of $234,617.
Our continuation as a going concern is dependent upon our ability to obtain necessary financing to continue operations and the attainment of profitable operations. As of September 30, 2024, we have incurred an accumulated deficit of $70,212,617 and have not yet generated any revenue from operations. Management anticipates that our cash on hand and funds that may be raised pursuant to the July Sales Agreement and $8 million grant from CIRM will be sufficient to fund planned operations for at least 12 months from the filing date of this Quarterly Report on Form 10-Q.
| 32 |
We will have additional capital requirements going forward and may need to seek additional financing, which may or may not be available to us on acceptable terms, if at all.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act (the “JOBS Act”) was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have chosen to take advantage of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards until those standards would otherwise apply to private companies provided under the JOBS Act. As a result, our financial statements may not be comparable to those of companies that comply with public company effective dates for complying with new or revised accounting standards.
Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including, without limitation, (i) providing an auditor’s attestation report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, as amended, and (ii) complying with the requirement adopted by the Public Company Accounting Oversight Board regarding the communication of critical audit matters in the auditor’s report on financial statements. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of our initial public offering (December 31, 2026); (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
Critical Accounting Policies and Use of Estimates
Our financial statements are prepared in accordance with U.S. GAAP. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. Management regularly evaluates its estimates and judgments, including those related to revenue recognition, intangible assets, long-lived assets valuation, variable interest entities, and legal matters. Actual results may differ from these estimates which may be material. “Note 2 – Summary of Significant Accounting Policies” in Part I, Item 1 of this Quarterly Report on Form 10-Q and in the Notes to Consolidated Financial Statements in Part II, Item 8 of our Annual Report on Form 10-K for the year ended December 31, 2023 (the “2022 Form 10-K”), and “Critical Accounting Policies” in Part II, Item 7 of the 2023 Form 10-K describe the significant accounting policies and methods used in the preparation of the Company’s financial statements. There have been no material changes to the Company’s critical accounting policies and estimates since the 2023 Form 10-K.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are not required to provide the information required by this Item as we are a “smaller reporting company,” as defined in Rule 12b-2 of the Exchange Act.
| 33 |
ITEM 4. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our “disclosure controls and procedures” (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) as of September 30, 2024, the end of the period covered by this Quarterly Report on Form 10-Q. The term “disclosure controls and procedures” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files under the Exchange Act is accumulated and communicated to a company’s management, including its principal executive officer and principal financial officer, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected. Based on the evaluation of our disclosure controls and procedures as of September 30, 2024, our management, with the participation of our principal executive officer and principal financial officer has concluded that, based on such evaluation, as of the end of the period covered by this Quarterly Report on Form 10-Q, our disclosure controls and procedures were not effective due to the material weakness described below.
Material Weakness in Internal Controls Over Financial Reporting
We identified a material weakness in our internal control over financial reporting that existed as of December 31, 2023. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. We determined that we had a material weakness because, due to our small size, and our limited number of personnel, we did not have in place an effective internal control environment with formal processes and procedures, including adequate segregation of duties within account processes and systems, and journal entry processing and review, to allow for a detailed review of accounting transactions that would identify errors in a timely manner. Based on our assessment, our management concluded that, as of September 30, 2024, the material weakness still exists.
Notwithstanding the material weaknesses in our internal control over financial reporting, we have concluded that the condensed consolidated financial statements included in this Quarterly Report on Form 10-Q fairly present, in all material respects, our financial position, results of operations and cash flows for the periods presented in conformity with accounting principles generally accepted in the United States of America.
Management’s Plan to Remediate the Material Weakness
We are committed to continually improving our internal controls over financial reporting. Subsequent to December 31, 2023, we appointed a new vice president of finance and accounting and director of corporate strategy, as part of our program to develop and implement effective internal controls over financial reporting. Additionally, management is currently working on the plan to address the material weaknesses noted above.
The material weaknesses will not be considered remediated, however, until the applicable controls operate for a sufficient period and management has concluded, through testing, that these controls are operating effectively. As we continue to evaluate and work to improve our internal control over financial reporting, we may decide that additional measures are necessary to address these identified control deficiencies.
Changes in Internal Control
Other than the remediation actions noted above, there have been no changes in our internal control over financial reporting that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| 34 |
PART II — OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS.
From time to time, we may become involved in various lawsuits and legal proceedings, which arise in the ordinary course of business. Litigation is subject to inherent uncertainties and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating results.
ITEM 1A. RISK FACTORS.
Risk factors that affect our business and financial results are discussed in Part I, Item 1A “Risk Factors,” in our Annual Report on Form 10-K for the year ended December 31, 2023 (“Annual Report”) as filed with the SEC on March 29, 2024 and below. There have been no material changes in our risk factors from those previously disclosed in our Annual Report, except as set forth below. You should carefully consider the risks described in our Annual Report and below, which could materially affect our business, financial condition or future results. The risks described in our Annual Report are not the only risks we face. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, and/or operating results. If any of the risks actually occur, our business, financial condition, and/or results of operations could be negatively affected.
Economic uncertainty may affect our access to capital and/or increase the costs of such capital.
Global economic conditions continue to be volatile and uncertain due to, among other things, consumer confidence in future economic conditions, fears of recession and trade wars, the price of energy, fluctuating interest rates, the availability and cost of consumer credit, the availability and timing of government stimulus programs, levels of unemployment, increased inflation, tax rates, and the war between Ukraine and Russia which began in February 2022, and Israel and Hamas, which began in October 2023 and which threatens to spread to other Middle Eastern countries. These conditions remain unpredictable and create uncertainties about our ability to raise capital in the future. In the event required capital becomes unavailable in the future, or more costly, it could have a material adverse effect on our business, future results of operations, and financial condition.
Our outstanding options and warrants may adversely affect the trading price of our securities.
As of September 30, 2024, we had (i) outstanding stock options to purchase an aggregate of 4,010,988 shares of common stock at a weighted average exercise price of $2.03 per share; (ii) outstanding Pre-Funded warrants to purchase 1,913,661 shares of common stock with an exercise price of $0.0001; and (iii) outstanding warrants to purchase 397,500 shares of common stock with a weighted average exercise price of $4.11 per share (when not including the Pre-Funded warrants). For the life of the options and warrants, the holders have the opportunity to profit from a rise in the market price of our common stock without assuming the risk of ownership. The issuance of shares upon the exercise of outstanding securities will also dilute the ownership interests of our existing stockholders.
The availability of these shares for public resale, as well as any actual resales of these shares, could adversely affect the trading price of our common stock. We cannot predict the size of future issuances of our common stock pursuant to the exercise of outstanding options or warrants or conversion of other securities, or the effect, if any, that future issuances and sales of shares of our common stock may have on the market price of our common stock. Sales or distributions of substantial amounts of our common stock (including shares issued in connection with an acquisition), or the perception that such sales could occur, may cause the market price of our common stock to decline.
In addition, the common stock issuable upon exercise/conversion of outstanding convertible securities may represent overhang that may also adversely affect the market price of our common stock. Overhang occurs when there is a greater supply of a company’s stock in the market than there is demand for that stock. When this happens the price of our stock will decrease, and any additional shares which stockholders attempt to sell in the market will only further decrease the share price. If the share volume of our common stock cannot absorb shares sold by holders of our outstanding convertible securities, then the value of our common stock will likely decrease.
| 35 |
A significant number of our shares are eligible for sale and their sale or potential sale may depress the market price of our common stock.
Sales of a significant number of shares of our common stock in the public market could harm the market price of our common stock. Most of our common stock is available for resale in the public market, including (a) outstanding stock options to purchase an aggregate of 4,010,988 shares of common stock at a weighted average exercise price of $2.03 per share; (b) Pre-Funded warrants to purchase 1,913,661 shares of common stock with an exercise price of $0.0001; and (c) 3,241,076 shares of common stock, the resale of which has been registered under the Securities Act. We have also filed certain Form S-8 Registration Statements pursuant to which we can issue unregistered stock in connection with awards under our equity plans. If a significant number of shares were sold, such sales would increase the supply of our common stock, thereby potentially causing a decrease in its price. Some or all of our shares of common stock, including those discussed above, may be offered from time to time in the open market pursuant to effective registration statements and/or compliance with Company insider trading policy, Exchange Act Section 16 and/or Rule 144, which sales could have a depressive effect on the market for our shares of common stock. Subject to certain restrictions, a person who has held restricted shares for a period of six months may generally sell common stock into the market. The sale of a significant portion of such shares when such shares are eligible for public sale may cause the value of our common stock to decline in value.
We may not receive the $8 million which we recently learned was granted to us by the California Institute for Regenerative Medicine.
On July 25, 2024, the Company learned that it was awarded an $8 million grant from the California Institute for Regenerative Medicine (CIRM) to support the clinical development of chimeric antigen receptor T-cell therapy NXC-201 for the treatment of relapsed/refractory AL Amyloidosis. The award is payable to the Company upon achievement of milestones that are primarily based on patient enrollment in the Company’s clinical trials. Additionally, if CIRM determines, in its sole discretion, that the Company has not complied with the terms and conditions of the grant, CIRM may suspend or permanently cease disbursements. Funds received under this grant may only be used for allowable project costs specifically identified with the CIRM-funded project. Such costs can include, but are not limited to, salary for personnel, itemized supplies, consultants, and itemized clinical study costs. Under the terms of the grant, both CIRM and the Company will co-fund the research project and the amount of the Company’s co-funding requirement is predetermined as a part of the award. The Company signed the grant agreement in November 2024 and expects to begin receiving funds from the grant beginning in November of 2024. The Company has not yet received any funds in connection with such award and may not receive funds on a timely basis, or at all, and such award may come with conditions. The Company is required to complete certain requirements and agree to certain terms and conditions in connection with such grant, which have not been completed or agreed to as of the date of this Report. In the event the award was not received on a timely basis, or at all, or subject to conditions, the Company could be forced to seek out alternative funding which may not be on as favorable terms as such currently expected grant.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS.
Unregistered Sales of Equity Securities
During the three months ended September 30, 2024, the Company issued 19,737 shares of restricted common stock valued at $37,500 for investor relations services based on the closing price on the date of the agreement pursuant to a marketing services agreement entered into on September 20, 2024.
During the three months ended September 30, 2024, the Company issued 31,641 shares of restricted common stock valued at $67,500 for investor relations services based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered into on July 25, 2023.
Subsequent to September 30, 2024, the Company issued 27,062 shares of restricted common stock valued at $45,000 for investor relations services based on the average closing price for the prior 10 trading days pursuant to a marketing services agreement entered into on July 25, 2023.
The issuances described above were exempt from registration pursuant to Section 4(a)(2), and/or Rule 506 of Regulation D of the Securities Act, since the foregoing issuances did not involve a public offering, the recipient took the securities for investment and not resale, we took take appropriate measures to restrict transfer, and the recipient was (a) an “accredited investor”; and/or (b) had access to similar documentation and information as would be required in a Registration Statement under the Securities Act. The securities are subject to transfer restrictions, and the securities contain an appropriate legend stating that such securities have not been registered under the Securities Act and may not be offered or sold absent registration or pursuant to an exemption therefrom. The securities were not registered under the Securities Act and such securities may not be offered or sold in the United States absent registration or an exemption from registration under the Securities Act and any applicable state securities laws.
| 36 |
ITEM 5. OTHER INFORMATION.
Rule
10b5-1 Trading Plans. During the quarter ended September 30, 2024, none of the Company’s directors or officers (as defined
in Rule 16a-1(f))
ITEM 6. EXHIBITS.
| * | Filed herewith. |
| ** | Furnished herewith. |
| 37 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| IMMIX BIOPHARMA, INC. | ||
| Date: November 12, 2024 | By: | /s/ Ilya Rachman |
| Ilya Rachman | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| Date: November 12, 2024 | By: | /s/ Gabriel Morris |
| Gabriel Morris, | ||
| Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | ||
| 38 |