美國證券交易委員會
華盛頓, DC 20549
形式
(馬克·奧內爾)
| | 針對部分的年度報告 13或15(d) 1934年證券交易所法案 | |
| 財政 止年度 | ||
| 或 | ||
| | 根據部分的過渡報告 13或15(d) 1934年證券交易所法案 | |
| 的過渡期 至 . |
委員會文件號:
阿森塔, Inc.
(註冊人章程中規定的確切名稱)
| | | |
| (述明或其他司法管轄權 公司或組織) | (稅務局僱主 識別號碼) | |
| (主要行政辦公室地址) | (郵政編碼) | |
註冊人的電話號碼,包括區號: (
根據第32條登記的證券 12(b) 該法案的:
| 每個班級的標題 | 交易符號 | 註冊的每個交易所的名稱 |
| | | 這個 |
根據第32條登記的證券 12(g) 該法案的:
沒有一
如果註冊人是《證券法》第405條所定義的知名經驗豐富的發行人,則通過勾選標記進行驗證。 是的
如果註冊人無需根據1934年證券交易法第13或15(d)條提交報告,則用複選標記進行驗證。 是的
通過勾選標記標明註冊人是否(1)在過去12個月內(或在註冊人被要求提交此類報告的較短期限內)提交了1934年證券交易法第13或15(d)條要求提交的所有報告,以及(2)在過去90天內是否已遵守此類提交要求。
通過勾選標記檢查註冊人是否已在過去12個月內(或在註冊人被要求提交此類文件的較短期限內)以電子方式提交了根據S-t法規第405條(本章第232.405條)要求提交的所有交互數據文件。
通過複選標記來確定註冊人是大型加速申報人、加速申報人、非加速申報人、小型報告公司還是新興成長型公司。請參閱《交易法》第12 b-2條中「大型加速申報人」、「加速申報人」、「小型報告公司」和「新興成長型公司」的定義。
| | 加速的文件服務器☐ |
| 非加速文件服務器☐ | 規模較小的報告公司 |
| 新興成長型公司 |
如果是新興成長型公司,用複選標記表示註冊人是否已選擇不使用延長的過渡期來遵守根據《交易法》第13(A)節提供的任何新的或修訂的財務會計準則。-☐
用複選標記表示註冊人是否提交了一份報告,證明其管理層根據《薩班斯-奧克斯利法案》(《美國聯邦法典》第15編,第7262(B)節)第404(B)條對其財務報告的內部控制的有效性進行了評估,該評估是由編制或發佈其審計報告的註冊會計師事務所進行的。
如果證券是根據該法第12(B)條登記的,應用複選標記表示登記人的財務報表是否反映了對以前發佈的財務報表的錯誤更正。
用複選標記表示這些錯誤更正中是否有任何重述需要對註冊人的任何執行人員在相關恢復期間根據第240.10D-1(B)條收到的基於激勵的補償進行恢復分析。
通過勾選標記檢查註冊人是否是空殼公司(定義見《交易法》規則12 b-2)。 是的
截至2024年3月28日,註冊人的非附屬公司持有的註冊人普通股的總市值約爲0.01美元,面值約爲美元
以引用方式併入的文件
登記人爲2025年股東年度會議提交的涉及董事選舉的部分委託聲明預計將在登記人財年結束後120天內提交,並以引用方式納入本年度報告第三部分10-k表格。
與前瞻性陳述相關的信息
本年度報告中的10-k表格包含前瞻性陳述,這些陳述屬於或可能被視爲符合1995年《私人證券訴訟改革法》(經修訂)、《1933年證券法》(經修訂)第27A節或《證券法》、《1934年證券交易法》(經修訂)或《交易法》所指的前瞻性陳述。所有非歷史事實的陳述,包括關於我們的信念或期望的陳述,都是前瞻性陳述。這些陳述可以用諸如「預期」、「估計」、「打算」、「相信」、「預期」、「可能」、「將」、「應該」、「可能」、「繼續」、「可能」或類似的陳述或此類術語的變體等前瞻性術語來識別。前瞻性陳述包括但不限於與我們未來的收入、利潤率、成本、運營費用、稅費、資本支出、收益、盈利能力、產品開發、需求、接受度和市場份額、競爭力、市場機會和業績、研發水平、我們的營銷、銷售和服務努力的成功、外包活動、預期的製造、客戶和技術要求、我們提供的解決方案的持續可行性和客戶的成功、我們管理層對我們當前和未來運營和業務重點的計劃和目標、法律訴訟、我們留住、聘用和整合技術人員的能力等有關的陳述。我們識別和應對增加的網絡安全風險的能力,包括員工繼續遠程工作的結果,我們業務的預期增長前景,預期收益和與我們的資產剝離和收購有關的其他陳述,成本節約計劃和我們的業務轉型計劃的充分性、有效性和成功,我們繼續識別收購目標併成功收購和整合理想的產品和服務並實現預期收入和收入協同效應的能力,我們採用新發布的會計指導,客戶支出水平,我們對關鍵供應商或供應商的依賴,以可接受的條件爲我們的業務獲得服務,包括供應鏈中斷的影響,總體經濟狀況,通脹的影響,支持未來運營的財務資源充足,以及我們對財務報告的內部控制存在明顯的實質性弱點。此類表述基於當前預期,涉及風險、不確定因素和其他因素,可能導致實際結果、我們的業績或我們的成就與此類前瞻性表述明示或暗示的任何未來結果、業績或成就大不相同。此類因素包括本10-k表格年度報告第I部分第1A項「風險因素」以及我們不時提交給美國證券交易委員會或美國證券交易委員會的其他文件,如我們的10-Q表格季度報告和我們當前的10-k表格報告。敬請讀者不要過度依賴這些前瞻性陳述,這些陳述僅在本文發佈之日發表,並基於我們目前和合理了解的信息。我們不承擔任何義務發佈對這些前瞻性陳述的修訂,以10-k表格的形式反映本年度報告日期之後發生的事件或情況,或反映預期或意外事件的發生或影響。本文中所作的預防性陳述應理解爲適用於所有相關前瞻性陳述,無論它們出現在本年度報告的10-k表格中。
除非上下文另有說明,否則本年度報告表格10-k中提及的「我們」、「我們」、「我們的」、「公司」和其他類似提及均指Azenta,Inc.及其合併子公司。
商標、商號和服務標誌
這份10-k表格年度報告包括我們的商標、商品名稱和服務標記,這些都是我們的財產,受適用知識產權法的保護。僅爲方便起見,商標、商品名稱和服務標記可能會出現在本年度報告中,表格10-k,但不包括 ®, TM 和 SM但此類引用並不意味着我們或適用所有人在適用法律允許的最大範圍內放棄或不會主張我們或任何適用許可人對這些商標、商號和服務標記的權利。我們無意使用或展示其他方的商標、商號或服務標誌,並且此類使用或展示不應被解釋爲暗示與這些其他方有關係,或由這些其他方背書或贊助我們。
行業和其他數據
除非另有說明,否則本年度報告中包含的有關我們的行業和我們經營的市場的信息,包括我們的一般預期、市場地位和市場機會,都是基於管理層的估計和研究,以及行業和一般出版物以及由第三方進行的研究、調查和研究。我們相信本年報所載的這些第三方刊物、研究、調查和研究的資料是可靠的。管理層的估計是根據可公開獲得的信息、他們對我們行業的了解以及他們基於這些信息和知識的假設得出的,我們認爲這些信息和知識是合理的。這些數據涉及許多假設和限制,由於各種因素,這些假設和限制必然會受到高度不確定性和風險的影響,包括在本年度報告中以10-k表格形式在上文「與前瞻性陳述有關的信息」項下和在下文第I部分第1A項「風險因素」下所描述的那些因素,以及在隨後提交給美國證券交易委員會的文件中更新和/或補充的那些因素。這些因素和其他因素可能會導致我們未來的業績與我們的假設和估計大不相同。
概述
我們是爲生命科學行業提供生物和化學化合物樣品探索和管理解決方案的全球領先供應商。我們於2011年進入生命科學市場,利用我們當時在半導體制造市場應用的內部精密自動化和低溫技術能力。這導致我們開發和提供自動化超冷存儲解決方案。自那以來,我們通過內部投資和一系列收購擴大了我們的生命科學產品。我們現在通過我們的樣本管理、自動化存儲、疫苗冷藏和運輸以及基因組服務專業知識爲我們的客戶提供從研究和臨床開發到商業化的支持,以及基因組服務專業知識,以幫助我們的客戶更快地將有效和突破性的療法推向市場。我們理解樣本完整性的重要性,並在樣本生命週期的每個階段爲客戶提供廣泛的產品和服務,包括採購、自動化存儲系統、基因組服務和大量樣本消耗品、信息學和數據軟件,以及樣本庫服務。我們的專業知識、全球足跡和領導地位使我們成爲製藥、生物技術和生命科學研究機構值得信賴的全球合作伙伴。截至2024年9月30日,我們在全球總共僱傭了約3,300名全職員工、兼職員工和臨時工,在大約125個國家和地區擁有銷售業務。我們的總部設在馬薩諸塞州伯靈頓,業務遍及北美、亞洲和歐洲。
我公司成立於1978年,已成爲全球半導體制造行業領先的自動化提供商和合作夥伴。2022年2月,我們以29億美元現金剝離了最後一項半導體業務,此後僅作爲一家生命科學公司運營。2021年12月1日,我們將公司名稱從「Brooks Automation,Inc.」更改。致「Azenta,Inc.」我們的普通股開始在納斯達克全球精選市場交易,代碼爲「AZTA」。半導體自動化業績被歸類爲已終止業務,除非另有說明,本年度報告中對10-k表格的業務的描述僅與我們的持續業務有關。
我們的產品組合包括我們內部開發以及通過收購獲得的產品和服務,旨在爲我們的客戶提供全面的能力,滿足他們在樣本探索和管理、自動化存儲、多元組學和冷鏈解決方案方面的需求。我們繼續開發新的產品和服務,並通過我們研發資源的專業知識增強現有和收購的產品。我們相信,我們的收購、投資和集成方法使我們能夠加速內部開發,並顯着加快生命科學解決方案的上市時間。
有關我們收購的更多信息,請參閱注4, 企業合併 本年度報告第二部分第8項「財務報表和補充數據」包含的合併財務報表(表格10-k)。
生命科學市場
我們的業務爲生命科學行業的廣泛終端市場提供服務,幫助我們的客戶推進治療方法的開發,以改善人們的生活和治癒疾病。隨着生物製品和個性化藥物的出現,生物樣本已成爲藥物和治療管道成功的關鍵資產,對這些樣本的適當管理和保護對我們的客戶來說非常重要。因此,我們相信我們有相當大的市場機會提供全面的樣本管理和基因組解決方案。
自本世紀之交成功繪製完整人類基因組以來,基因組服務市場不斷增長,以支持生物藥物開發、個性化醫療以及細胞和基因療法(CGt)的研究。頂級製藥和生物技術公司和機構可以利用其內部實驗室資源對數百萬個基因進行測序,作爲其研究工作流程的一部分。然而,許多公司和機構希望將全部或部分基因測序外包給提供快速結果和專家諮詢服務的獨立實驗室。我們作爲增值實驗室服務提供商參與這一市場,提供高質量的基因檢測服務、快速週轉時間和專家客戶支持。
我們在全球擁有約14,000名客戶,相信我們有能力擴大我們的客戶群。我們爲頂級製藥和生物技術公司、進行臨床研究和治療開發的最先進的研究醫院以及生物技術領域的一些最新和領先的初創企業提供服務。此外,我們還爲學術和政府機構提供服務。我們相信,基於樣本的服務和產品業務將繼續展現出增長軌跡。
細分市場
自2023年10月1日起,我們將組織結構重新調整爲三個主要業務部門,以增強我們加速增長的商業戰略並實現額外的盈利計劃。這些分部與我們的首席運營決策者(「CODM」)管理業務、分配資源和評估績效的方式的變化保持一致。我們的經營和可報告分部包括以下部分:
| ● |
樣本管理解決方案.樣本管理解決方案業務資源作爲一個單一業務部門運營,提供端到端樣本管理產品和服務,包括樣本儲存庫服務(SAS)和核心產品(自動化商店、低溫系統、自動試管樣本、消耗品和儀器以及受控速率解凍設備)。 |
|
| ● | 多元經濟學. Multiomics業務資源作爲一個單一業務部門運營,提供基因組和其他樣本分析服務,包括基因測序、合成編輯和相關服務。 |
| ● |
b醫療系統. b醫療系統業務資源作爲一個單一業務部門運營,專注於在國際市場上向政府、衛生機構和非政府組織製造和分銷溫控存儲和運輸解決方案。 |
有關我們的可報告和經營分部的更多信息,請參閱附註18, 細分市場和地理信息 本年度報告第二部分第8項「財務報表和補充數據」包含的合併財務報表(表格10-k)。
樣本管理解決方案
在我們的樣品管理解決方案部門中,我們作爲一個單一的業務部門提供端到端的樣品管理產品和服務,包括SRS和核心產品(自動化倉庫、低溫系統、自動樣品管、消耗品和儀器、冷凍和受控速率解凍設備)。該產品組合在樣品的整個生命週期中爲客戶提供高水平的樣品質量、安全性、可用性、智能性和完整性,爲客戶提供完整的端到端「冷鏈保管」能力。我們還爲客戶提供整個實驗設計和實施過程的專家級諮詢服務。2022年7月1日,我們收購了Barkey Holding GmbH及其子公司「Barkey」,爲醫療、生物技術和製藥行業的客戶提供受控速率解凍設備的領先供應商。此次收購增加了創新的產品和能力,延長了我們廣泛的冷鏈條件產品和服務組合,同時還擴大了我們在快速增長的CGT領域的客戶覆蓋範圍。
SRS - 我們的SAS服務包括一系列完整的服務,包括現場和場外樣本儲存、冷鏈物流、樣本運輸和收集搬遷、生物處理解決方案(包括樣本製備和基於實驗室的樣本分析)、災難恢復和業務連續性,以及項目管理和諮詢。我們的信息化解決方案提供樣本智能軟件解決方案,並支持生命科學工具和儀器工作單元的實驗室工作流程安排、樣本庫存和物流、環境和溫度監測、臨床試驗和同意管理以及樣本收集的規劃、數據管理、虛擬化和可視化。我們爲客戶日益分散的工作流程提供增強的生物樣本庫存現場和非現場管理和集成解決方案。
自動化商店 - 我們的自動化商店產品包括獨立系統,可在環境溫度至-80 ° C到-190 ° C低溫存儲的溫度範圍內存儲超過2000萬個樣本。我們的自動化商店具有獨特的設計,允許將溫度控制在-80 ° C,具有行業最高的樣本提取吞吐量。我們的自動化存儲庫提供高通量能力和多規格試管和板的優化存儲,同時在存儲的樣本中保持一致的溫度曲線。我們還提供一系列服務,旨在優化存儲產品的生產力。
低溫系統 - 我們的低溫系統產品提供低溫存儲,從高效液氮蒸氣低溫冷凍機到保持樣本完整性和監管鏈的全自動系統,以及在冷鏈的每個步驟中保持樣本安全所需的存儲材料。我們的低溫系統提供長期低溫存儲,具有準確的記錄保存和可靠的溫度控制,即使在運輸過程中也是如此。這些系統將記錄良好的樣本保護和全面的庫存管理與卓越的用戶體驗相結合,使我們的客戶能夠規劃可擴展的低溫基礎設施以維持質量和文檔。
自動樣本管 - 我們的自動化樣本管產品包括一系列自動化友好型存儲管,具有編碼選項,例如2D編碼、雙編碼和三編碼,具有外螺紋或內螺紋,以及用於更快讀取、封蓋和去封蓋的儀器。
消耗品和儀器 - 我們的消耗品和儀器產品包括全系列的消耗品,包括多種格式的架子、管子、蓋子、板和箔,用於在環境至超冷存儲環境中存儲和處理樣本。用於貼標籤、條形碼、封蓋、去蓋、審核、密封、剝離和刺穿管子和板的全面儀器是我們的消耗品的補充。我們的產品包括一系列針對基因組樣本製備和服務市場的產品,包括聚合酶鏈反應(PCR)、測序、成像、板密封、液體處理和樣本處理。
受控速率解凍裝置 - 我們的受控速率解凍設備包括一系列用於自動解凍血漿、血液和幹細胞以及CGt應用的產品。我們的產品用於冷凍保存樣本和療法的受控速率解凍,並用於研發、臨床試驗、良好製造規範和醫院環境。
多元經濟學
基因組服務 - 我們的基因組服務業務包括基因測序和基因合成服務,能夠擴大基於基因的醫療保健發現和療法的研究和開發。這些服務包括下一代測序(NGS)、Sanger測序、基因合成、生物信息學和良好實驗室規範(GLP)監管服務。測序服務提供提取、庫製備、測序和生物信息學的標準和定製服務,由博士支持級別項目經理提供諮詢服務、更新和交付後協助。我們的基因合成產品提供廣泛的序列長度和結構複雜性的生產、DNA克隆、基因片段合成、寡聚物合成和載體純化。
b醫療系統
在我們的b醫療系統部門,我們提供溫控存儲和運輸解決方案,補充我們的冷鏈能力,爲全球範圍內可靠且可追溯地運輸溫度敏感樣本添加差異化的解決方案。我們通過冷鏈運輸解決方案、血漿冷凍機、接觸式休克冷凍機、超低冷凍機以及實時樣本監測和位置跟蹤解決方案的產品組合,爲疫苗、血液成分和實驗室樣本提供端到端的冷鏈監管能力。
溫控儲存和運輸解決方案 - 我們的溫控存儲和運輸解決方案能夠向全球150多個國家提供救生治療。這些產品補充了我們的冷鏈能力,爲可靠且可追溯的溫度敏感樣本運輸添加了差異化的解決方案。
銷售、營銷和客戶支持
我們的大部分銷售都是通過我們的直銷隊伍完成的,特別是我們的商店系統、存儲服務和基因組服務。我們通過分銷商補充消耗品和儀器的銷售,覆蓋廣泛的客戶。在新興生命科學產業的地區,我們利用當地分銷商協助自動化商店的銷售流程,並利用包括聯合國兒童基金會在內的國際採購機構的能力。
我們的SAS和更大的自動化商店系統的銷售流程需要數月時間才能完成,並且可能涉及銷售、營銷和工程團隊。基因組服務的銷售通常通過客戶實驗室的在線訂單產生,並使用快遞服務向客戶交付,最簡單的基因組學和合成請求在不到24小時內完成,更復雜的項目在數週內完成。
參加貿易展覽、研討會和行業論壇只是我們營銷舉措的一小部分。我們還製作和分發銷售手冊、網絡研討會和白皮書,並在商業和工業出版物上發佈新聞稿和文章。我們在亞洲、歐洲、中東和北美設有銷售和服務中心,以加強對客戶的支持和溝通。
我們通常根據產品類型提供一到五年的產品保修,其中一些太陽能冷鏈產品的保修期長達十年,因爲它們與實時監控服務相連。客戶支持能力包括利用場外技術人員和當地代理提供的國內支持。
競爭
鑑於我們的樣本管理解決方案、多組學和醫療系統部門提供的解決方案和服務的廣度,我們相信我們擁有獨特的產品和服務組合。然而,這三個細分市場中的每一個在其產品領域都有獨特的競爭對手。在樣本管理解決方案部門,我們的主要競爭對手包括自動化系統的Hamilton Company和Licic AG以及美國實驗室控股公司和賽默飛世科學公司。用於存儲、消耗品和服務。在多元組學領域,我們的主要競爭對手包括華大基因公司、有限公司,Eurofins,科學SE,GenScript Biotech Corporation、Integrative DNA Technologies,Inc.、諾金公司,有限公司,和Twist Bioscience Corporation。在b醫療系統部門,我們的主要競爭對手包括Vestfrost Solutions和Haier Biomedical。
研究與開發
我們的研發工作重點是開發新產品並增強現有產品和服務的功能、集成度、可靠性和性能。我們的工程、營銷、運營和管理人員利用與客戶組織中同行的密切合作關係,主動識別市場需求,幫助我們重新調整研發投資的重點,以滿足客戶的需求。
我們已經開發並繼續開發用於超低溫環境下運行的自動化生物樣本存儲解決方案。我們擁有從環境溫度到零下190°C的完整的自動化存儲系列。我們的自動存儲系統爲疫苗和生物製品提供改進的數據管理和樣本安全性,並具有獨特的設計,允許將溫度控制在-80°C以下,並具有業界最高的樣本提取吞吐量。我們的基因組服務促進了基因測序、合成、編輯和相關服務的研發活動,以滿足市場需求。我們投資於研發,在我們自己的實驗室開發協議和效率,並向我們的客戶提供專有產品。例如,在我們的多組學部門,我們通過增加對腺相關病毒(CGT中使用的一種常見載體)的分析的受監管服務來豐富我們的產品組合。此外,我們繼續通過擴大我們的產品組合以包括蛋白質組學解決方案來增加藥物發現和開發研究的價值。我們將繼續專注於開發能夠簡化從樣本到數據的工作流程的流程和技術。
製造業和服務
我們的製造業務包括產品組裝、集成和測試。我們實施質量保證程序,包括標準設計實踐、可靠性測試和分析、供應商和零部件選擇程序、供應商控制、製造過程控制以及確保產品高質量性能的服務流程。我們的主要製造工廠位於美國密蘇里州、英國曼徹斯特和沃頓以及盧森堡霍辛根。我們的製造業務旨在通過響應靈敏且靈活的流程和採購策略在短時間內爲客戶提供高質量、最佳成本、差異化的產品。我們在大部分製造中利用精益製造技術。
我們在客戶附近設有服務和支持地點,以快速響應他們的服務需求。我們的主要產品服務和支持地點包括馬薩諸塞州伯靈頓和英國曼徹斯特。
我們在馬薩諸塞州比勒裏卡;印第安納州印第安納波利斯和普蘭菲爾德;加利福尼亞州弗雷斯諾;俄亥俄州克利夫蘭;德國格里斯海姆;加拿大蒙特利爾;新加坡;中國北京和美國各地的多個地點提供樣本管理存儲和運輸服務。我們擁有一個由13個提供基因組服務的實驗室網絡,其中美國6個、中國3個、英國2個、日本和德國各1個。
專利和專有權利
我們依靠專利、商業祕密法、保密協議和程序、版權、商標和許可協議來保護我們的技術。由於生命科學和相關過程設備行業的快速技術變革,我們認爲,現有技術的改進、對商業祕密、未獲得專利的專有知識的依賴以及新產品和服務的開發在建立和保持競爭優勢方面可能與專利保護一樣重要。我們的政策是要求所有員工簽訂專有信息和保密協議,以保護商業祕密和專業知識。然而,我們無法保證這些努力將有意義地保護我們的商業祕密。
截至2024年9月30日,我們擁有約92項已頒發的美國專利,其中多項相應專利在外國司法管轄區頒發,約36項外國(非美國)專利頒發的專利。我們還有大約41項正在處理的美國專利申請,其中一些申請的外國同行已經提交或可能在適當的時間提交。我們的專利從2024年開始在不同日期到期,此後將持續到2042年。
環境事務和政府法規
環境法規
我們遵守美國和其他國家/地區有關環境事務以及員工安全和健康的各種法律和政府法規。美國影響我們的聯邦環境立法包括《資源保護和恢復法》、《清潔空氣法》、《清潔水法》、《安全飲用水法》和《綜合環境響應補償和責任法》。我們還受到職業安全與健康管理局(OSHA)有關員工安全和健康問題的監管。美國環境保護局或EPA、OSHA和其他聯邦機構有權頒佈對我們運營有影響的法規。
除了這些聯邦法律和法規外,各州還根據聯邦法規被授予一定的權力,並根據州法律對這些事務擁有管轄權。許多州和地方政府已採用環境和員工安全與健康法律法規,其中一些與聯邦要求類似。我們運營所在的外國司法管轄區也採用了類似的法律和法規。
其他法律法規
我們的運營還受美國和我們運營和開展業務的其他國家/地區的其他政府法規的約束。雖然我們的大多數產品不受監管,但我們b醫療系統部門的某些產品以及從巴基樣本管理解決方案部門的某些產品受FDA根據《聯邦食品、藥品和化妝品法案》監管,並且我們在Multiomics業務中的普洛斯監管服務需要對我們提供這些服務的實驗室進行認證和認證。
我們的業務還包括進出口活動,我們受到美國商務部、國務院和財政部執行的相關法律的約束。此外,我們的物流活動必須遵守交通部、聯邦航空管理局和類似外國機構的規則和法規。
我們相信,我們在所有重大方面都遵守了所有適用的環境、員工健康和安全以及其他政府法規,這種合規性沒有、也預計不會對我們的資本支出、競爭地位、財務狀況或運營結果產生不利影響。
人力資本
截至2024年9月30日,我們在全球範圍內總共僱用了約3,300名全職員工、兼職員工和臨時工,主要在美國。我們明白,我們的成功取決於才華橫溢的員工,而我們的人力資本管理實踐的重點是吸引和留住多元化且敬業的勞動力。
多樣性、公平性和包容性.我們致力於吸引、培養和保留多元化人才,涵蓋各個年齡、性別、性別認同、種族、性取向、身體能力、神經差異、種族、信仰和觀點。我們的目標是通過尋求知識、提高意識、培養敏感性、樹立尊重以及促進包容和團結來發展文化能力。我們約46%的員工性別多元化,40%的美國-員工認爲種族多元化。有關我們性別和種族多樣性的更多詳細信息,請在我們網站的環境、社會和治理(ESG)治理報告中找到。
員工敬業度.我們致力於培養一種讓每個員工都感到被重視的文化和環境。我們的成功在很大程度上取決於我們在整個組織中僱用和留住頂尖人才,主要重點是我們的管理團隊和直接與客戶互動的員工。我們與其他規模較小的公司以及我們的市場和其他行業的公司爭奪人才。
薪酬和福利。 爲了吸引和留住頂尖人才,我們專注於擁有多元化、包容性和安全的工作場所,同時提供有競爭力的薪酬、福利以及健康和保健計劃。我們的大多數員工還擁有激勵薪酬機會,主要側重於滿足財務、銷售、運營和/或以客戶爲中心的指標。此外,我們的長期股權薪酬旨在使管理層利益與股東利益保持一致,並鼓勵創造長期價值。
培訓與發展.我們提供培訓和學習機會、輪換分配機會和持續的績效反饋,以促進員工的發展。我們的學習文化建立在正式課程、實踐社區、點對點學習、體驗式開發、支持工具和持續評估之上。我們傾聽員工的意見,以更好地了解他們的培訓和發展需求,並確保我們的產品同時滿足技術學習和領導力發展。我們提供慷慨的學費報銷計劃,鼓勵員工攻讀與其當前或抱負職位相關的領域的本科和研究生學位。
員工健康與安全。 遵守環境、健康和安全(即EH & S)法律和法規是我們實施的EH & S計劃的基礎。作爲我們的EH & S計劃的一部分,我們:
| ● |
幫助建立一種強調安全操作、程序、行爲和態度的安全文化; |
| ● |
提供有關一般安全原則和特定工作要求的合規培訓; |
| ● |
通過衆多培訓活動,讓員工認識並履行他們的安全責任; |
| ● |
提供適當的個人防護裝備和安全使用該設備的培訓; |
| ● |
幫助確保所有員工了解周圍環境,並確保每個人都努力維護安全的工作場所; |
| ● |
爲各級員工(包括行政管理層)定期舉行每月全公司安全委員會會議;以及 |
| ● |
鼓勵員工進行工作危害分析,以識別工作場所危害並降低風險。 |
宗旨和核心價值觀.我們公司的目標是使世界各地的生命科學組織能夠更快地將有影響力和突破性的療法推向市場。我們致力於確保每個團隊成員都了解我們的核心價值觀:以客戶爲中心、成就、問責制、團隊合作、員工價值和誠信。這些核心價值觀是我們採取行動和決策的基礎,並體現在我們的行爲標準中,其中概述了我們對客戶、投資者、社區以及彼此的承諾。我們的行爲標準還概述了對員工的期望,並確保我們繼續培養高度誠信的文化。我們遵守聯邦和州法律、美國證券交易委員會和納斯達克全球精選市場制定的治理要求,努力建立適當的風險管理方法和控制程序,以充分管理、監控和控制我們日常可能面臨的重大風險。
可用信息
我們向SEC提交年度、季度和當前報告、代理聲明和其他信息。我們的SEC文件可在SEC網站www.example.com上向公衆提供。我們還維護一個網站www.azenta.com,您可以通過該網站訪問我們的SEC文件和我們的新聞稿副本。http://www.sec.gov我們網站上找到的信息不是我們向SEC提交或提供的本報告或任何其他報告的一部分。
因素可能 影響未來結果
在決定投資我們的普通股之前,您應仔細考慮以下描述的風險以及本10-k表格年度報告中的其他信息。這些是適用於我們業務的風險和不確定性,我們認爲您最需要考慮。我們目前尚不清楚的額外風險和不確定性,我們目前認爲這些風險和不確定性並不重大,或者與我們行業或業務中的其他公司面臨的風險類似,也可能損害我們的業務運營。如果實際發生以下任何風險或不確定性,我們的業務、財務狀況和經營業績可能會受到影響。在這種情況下,我們普通股的市場價格可能會下跌,您可能會失去全部或部分投資。
宏觀經濟和外部風險
宏觀經濟狀況長期低迷可能會對我們的業務產生重大不利影響。
美國和其他地區的經濟低迷、政府科研經費水平的減少、利率上升、通貨膨脹等因素可能會導致我們現有或潛在客戶推遲或減少購買,這反過來可能導致我們產品和服務的銷售額減少,對我們的運營業績和現金流產生實質性和不利的影響。全球金融市場的波動和中斷可能會限制我們的客戶獲得足夠的資金來維持運營和繼續執行計劃中的或新的資本支出計劃的能力,從而導致銷售額減少,這可能會對我們的運營業績和現金流產生實質性的不利影響。此外,由於經濟不景氣,客戶的支付能力下降,可能會增加應收賬款的收回難度,增加壞賬準備金和應收賬款的註銷,以及增加運營成本佔收入的百分比。
我們面臨與公共衛生威脅和流行病相關的風險。
我們面臨與公共衛生威脅和流行病相關的風險。公共衛生威脅,無論是否全球性,都可能對我們的業務和市場產生不利影響,包括我們的勞動力和運營以及我們客戶、供應商和業務合作伙伴的運營。特別是,我們可能會遭受重大財務或運營影響,包括:
| ● |
對我們的產品和/或服務的需求大幅波動或減少;或 |
| ● |
由於我們的運營或我們的第三方合作伙伴、供應商、承包商、物流合作伙伴或客戶的運營中斷而無法滿足我們客戶的需求或其他義務。 |
在我們和客戶運營的某些司法管轄區,這些影響可能會更嚴重,這些司法管轄區受到這些威脅的影響或採取更嚴格的政策應對威脅。雖然我們已經制定和實施並繼續制定和實施健康與安全協議、業務連續性計劃和危機管理協議,以努力減輕健康威脅對我們的員工和業務的負面影響,但無法保證我們的努力將取得成功,或者此類努力可能不會產生有害的意外後果,因此,我們的業務。財務狀況和運營結果可能會受到健康威脅和流行病的重大不利影響。
全球氣候變化以及相關法律和監管發展可能會對我們的業務、財務狀況和運營業績產生負面影響。
氣候變化給我們和我們的客戶帶來了風險,隨着時間的推移,風險預計會增加。我們的產品和服務受到美國聯邦、州和地方當局以及對我們的國際業務擁有管轄權的監管機構的環境法規的約束和影響。我們未來應對氣候變化的法規或自願行動可能會導致我們的設施發生代價高昂的變化,以減少碳排放,並可能因爲改用碳密集度較低但更昂貴的能源來運營我們的設施以及運輸和運輸產品和樣品而增加能源成本。不能保證氣候變化或環境監管和應對措施不會對我們提供樣本管理、自動化存儲和基因組服務的能力產生負面競爭影響,也不能保證經濟回報將與我們在開發新產品和服務方面的投資相匹配。我們可能會面臨與產品設計、受監管材料的使用、能源消耗和效率以及產品及其部件在使用結束或使用壽命結束時的重複使用、回收或處置有關的日益複雜的問題。仍然缺乏一致的氣候立法,這在未來的能效激勵和合規成本方面造成了經濟和監管方面的不確定性,這可能會影響對我們產品和服務的需求、我們與提供產品和服務相關的成本,以及我們的運營結果和財務狀況。此外,氣候變化對我們業務的潛在實際影響是高度不確定的,並將特定於我們業務所在地區的地理環境。這些可能包括全球天氣模式的變化,其中可能包括降雨和風暴模式和強度的局部變化、水資源短缺、海平面變化以及平均或極端溫度的變化。這些影響也可能對我們的財產、我們的業務、財務狀況和經營結果產生不利影響。
不利的貨幣匯率波動可能會影響我們持有的大量外幣,導致營業利潤率下降,或者可能導致我們提高產品和服務的價格,從而導致銷售額減少。
匯率波動可能會對我們的銷售額、銷售成本和經營結果產生不利影響,我們可能會在簽訂的遠期外匯合同方面蒙受損失。不利的匯率波動可能會要求我們提高產品和服務的價格,這可能會導致淨銷售額下降。或者,如果我們不針對不利的匯率波動調整產品和服務的價格,我們的經營結果,包括我們的利潤率,可能會受到實質性的不利影響。此外,我們的海外子公司的大部分銷售都是以銷售這些產品或提供這些服務的國家的貨幣計價的,由於匯率波動,支付此類銷售的貨幣可能會比收到時的美元價值更低。我們不時簽訂遠期外匯合約和交叉貨幣互換協議,以減少貨幣風險敞口。然而,我們不能確定我們的努力是否足以保護我們免受重大匯率波動的影響,或者這種努力是否不會使我們面臨額外的匯率風險,這可能會對我們的經營業績產生重大和不利的影響。
截至2024年9月30日,我們持有約1.24億美元的外幣現金和現金等值物,佔我們當前現金和現金等值物餘額的很大一部分。由於我們持有大量外幣,我們的財務業績和資本比率可能會受到匯率變動的影響,我們的很大一部分資產必須轉換爲美元以用於外部報告目的,或轉換爲美元以滿足我們的戰略需求和服務義務,例如任何未來以美元計價的債務或股息。我們可能會尋求減輕貨幣匯率波動的風險,但我們的努力可能不會成功。
我們的業務可能會受到環境、社會和治理(ESG)問題的負面影響。
投資者、客戶、員工和其他利益相關者越來越關注ESG事務,包括應對氣候變化,這可能會導致我們業務運營成本增加或限制我們活動的某些方面。衡量ESG工作和相關事項的標準正在發展和演變,某些領域受制於可能隨着時間的推移而變化的假設,而且氣候變化影響的程度和嚴重性尚不清楚。此外,我們可能會因此類舉措或目標的範圍而受到批評,或者被認爲在這些問題上不負責任。任何此類事項都可能對我們未來的經營業績、財務狀況和現金流產生重大不利影響。
與我們運營相關的風險
我們的經營業績可能會大幅波動,這可能會對我們的業務產生負面影響。
我們的收入、營業利潤率和其他經營業績可能會在季度和年度之間出現顯着波動,具體取決於多種因素,包括:
| ● |
由於我們的客戶集中或其他原因,我們客戶的產品訂單和服務合同的時間和條款發生變化; |
| ● |
對我們提供的產品和服務組合的需求變化; |
| ● |
我們普通股任何新回購的時間和金額; |
| ● |
我們新產品和服務推出的時機和市場接受度; |
| ● |
計劃推出新產品或服務,或交付給客戶後任何此類產品的性能或此類服務的質量出現延誤或出現問題; |
| ● |
我們的競爭對手的新產品、服務或技術創新,由於我們提供產品和服務的市場的快速技術變化,這可能會降低我們的產品和服務的競爭力; |
| ● |
任何收購、資產剝離或其他戰略交易的時間和相關成本; |
| ● |
我們有能力降低成本以應對對我們的產品和服務需求的下降; |
| ● |
我們準確估計客戶需求的能力,包括我們使用的需求預測的準確性; |
| ● |
我們的製造過程或零部件供應中斷; |
| ● |
過多或過時庫存的核銷; |
| ● |
有競爭力的定價壓力;以及 |
| ● |
增加對基礎設施的投資,以支持我們的增長,包括資本設備、研發以及銷售和營銷計劃,以支持持續的產品和服務創新、技術能力增強和銷售工作。創收的時機加上投資額的增加可能會導致運營虧損。 |
由於這些風險,我們認爲參考過去的業績來比較我們的收入和經營業績可能沒有意義,而且這些比較可能不是我們未來業績的準確指標。
如果我們不繼續及時有效地推出反映技術進步的新產品和服務,我們的產品和服務可能會過時,我們的經營業績也會受到影響。
我們的成功取決於我們應對所服務市場中存在的技術變化的能力。我們的產品開發以及向市場推出產品和服務的成功取決於我們的能力:
| ● |
以準確的方式識別和定義新的市場機會、產品和服務; |
| ● |
獲得市場對我們的產品和服務的接受; |
| ● |
及時且具有成本效益的方式創新、開發、獲取新技術和應用並商業化; |
| ● |
適應不斷變化的市場條件; |
| ● |
將我們的產品與競爭對手的產品區分開來; |
| ● |
必要時獲取和維護知識產權; |
| ● |
繼續制定全面、集成的產品和服務策略; |
| ● |
爲我們的產品和服務定價適當;以及 |
| ● |
按照高標準的可製造性設計我們的產品,以滿足客戶的要求。 |
如果我們無法成功地以及時且具有成本效益的方式應對技術和/或市場變化,或者如果我們推出的新產品和服務沒有獲得市場接受,我們的競爭地位將會減弱,這可能會對我們的業務和前景造成重大損害。
如果我們不能實現轉型計劃目標,我們的財務業績可能會受到負面影響。
在2024財年,我們宣佈了一項轉型計劃,旨在降低複雜性並簡化整個組織的流程,旨在降低成本並提高盈利能力。我們已經確定了支持轉型計劃目標的舉措和活動。如果我們未能完成任何這些舉措或活動,或者如果這些舉措和活動的結果未能帶來我們預期的成本節省,我們的財務業績可能會受到負面影響。
我們業務的全球性使我們面臨多重風險。
在截至2024年、2023年和2022年9月30日的財年,我們約44%、46%和33%的收入來自北美以外的銷售。我們預計,在可預見的未來,國際銷售,包括亞洲和非洲銷售額的增加,將繼續佔我們收入的很大一部分,特別是,我們對中國客戶的銷售比例將增加,這在很大程度上是由於我們在中國的重要基因組服務業務。此外,我們打算在中國的設施上投資額外資源,這將擴大我們在全球銷售、服務和維修業務的足跡。由於我們的國際業務,我們面臨許多風險和不確定性,包括:
| ● |
更長的銷售週期和收集時間; |
| ● |
關稅和國際貿易壁壘; |
| ● |
對國外知識產權和合同權的某些法律保護減少或減少; |
| ● |
我們運營所在司法管轄區不同且不斷變化的法律和監管要求; |
| ● |
政府貨幣管制和對收入匯回的限制; |
| ● |
多元化的勞動力隊伍,具有不同的經驗水平、語言、文化、習俗、商業實踐和工人期望,以及不同的就業實踐和勞工問題; |
| ● |
更多地依賴第三方代理和分銷商在我們沒有業務的司法管轄區進行業務交易; |
| ● |
外幣匯率和利率波動,特別是亞洲和歐洲; |
| ● |
我們業務所在地區的政治和經濟不穩定、變化、敵對行動和其他破壞;以及 |
| ● |
外國政府幹預或試圖控制我們的國際業務,包括中國政府幹預我們的中國蘇州工廠。 |
此外,在許多國家,特別是發展中經濟體國家,腐敗和/或賄賂的風險增加,這可能導致違反各種法律和法規,包括《反海外腐敗法》。 雖然我們的內部政策和程序禁止此類商業行爲,但無法保證我們的所有員工、承包商和代理人,以及我們將某些業務運營外包給的公司,將遵守這些政策和程序,或適用的反賄賂法律和法規。 任何此類違規行爲都可能使我們受到罰款和其他處罰,這可能會對我們的業務、經營業績、財務狀況和現金流產生重大不利影響。
一個或多個國家/地區任何這些領域的負面發展都可能導致對我們產品的需求減少、已下訂單的取消或延遲、對我們知識產權的威脅、收取應收賬款的困難以及開展業務的成本上升,其中任何一種都可能嚴重損害我們的業務和盈利能力。
截至2024年9月30日,我們在美國境外持有約1.46億美元現金,根據當地國家/地區的法定要求,我們將任何資金匯回美國或業務其他地方使用的能力可能會受到限制,這可能會對我們部署資本的機會產生負面影響。
如果我們未能充分整合我們已收購或可能收購的業務的運營,我們的業務可能會受到重大損害。
我們過去曾對具有補充產品、服務和/或技術的企業進行收購或重大投資,未來可能會進行。我們的收購帶來了許多風險,包括:
| ● |
難以整合被收購公司的運營、技術、產品和人員並實現合併後業務的預期協同效應; |
| ● |
定義和執行全面的產品和服務戰略; |
| ● |
管理進入我們直接經驗有限或沒有直接經驗的市場或業務類型的風險; |
| ● |
我們或被收購公司的關鍵員工、客戶和戰略合作伙伴的潛在流失; |
| ● |
意外問題或潛在責任,例如目標公司產品裝機量的質量問題或目標公司的活動、產品或服務侵犯另一家公司的知識產權; |
| ● |
與遵守被收購公司現有合同相關的問題; |
| ● |
難以管理地理分散的業務; |
| ● |
轉移管理層的注意力從企業正常日常運營上;以及 |
| ● |
難以準確估計對任何收購產品、服務或技術的預期需求及其時間和規律性。 |
如果我們收購新業務,我們可能會花費大量資金、產生額外債務或發行額外證券,這可能會對我們的運營產生負面影響並稀釋我們的股東。在收購後的時期內,我們將被要求評估聲譽和與收購相關的無形資產的損失。如果此類資產被發現出現損害,則將被減記至估計公允價值,並從收益中扣除。未能充分解決這些風險或任何資產的減損可能會對我們的業務和財務業績造成重大損害。
在當前市場內擴張會帶來新的競爭對手和商業風險。
我們增長戰略的一個關鍵部分是繼續在生命科學、產品和服務市場擴張。作爲這一戰略的一部分,我們希望通過利用我們的核心技術並收購特定的業務、產品、服務或技術來使我們的產品銷售和服務收入多樣化,這需要投資和資源,而這些投資和資源可能無法以有利的條款或根本得不到。我們不能保證我們將成功地利用我們的能力進入生命科學樣本管理和基因組服務市場,或識別併成功收購其他業務、產品、服務或技術,以滿足新客戶的所有需求,並與其他產品和服務展開有利競爭。由於我們增長潛力的很大一部分可能取決於我們在樣本管理解決方案、多重組學、醫療和b醫療系統各個細分市場增加銷售額的能力,因此我們無法在這些細分市場服務的市場內成功擴張可能會對未來的財務業績產生不利影響。
關鍵人員的變動可能會損害我們執行業務戰略的能力。
我們的高級管理人員和必要的工程、科學和管理人員的持續服務,以及我們吸引和留住這些人員的能力,是我們繼續執行我們的戰略的重要因素。在吸引這類員工方面存在着激烈的競爭,失去任何這類關鍵員工可能會對我們的業務和經營業績產生重大不利影響。如果工程和科學人員的流失率很高,而我們又無法取代他們,情況也可能是這樣。我們吸引和留住員工的能力可能會受到員工對我們與遠程工作相關的政策的反應的負面影響,特別是在美國。任何未能吸引、招聘、培訓、留住、激勵和整合合格人才,特別是我們新上任的總裁兼首席執行官兼執行副總裁總裁和首席財務官,都可能對我們的戰略計劃、經營業績和增長前景造成實質性損害。
約翰·馬洛塔(John Marotta)於2024年9月9日加入我們,擔任總裁、首席執行官和董事會成員,接替斯蒂芬·施瓦茨(Stephen Schwartz)博士,後者在爲我們服務超過14年後宣佈退休。此外,2024年11月12日,在提交本10-k表格年度報告後,Lawrence Link被任命爲我們的執行副總裁兼首席財務官,接替Herman Cueto擔任這一職務。儘管我們已採取措施幫助確保順利成功過渡,但無法保證這些步驟會成功。
意外事件可能會擾亂我們的樣本存儲操作,並對我們的聲譽和運營結果產生不利影響。
意外事件,包括我們設施的火災或爆炸、龍捲風、颶風和地震等自然災害、戰爭或恐怖活動、計劃外停電、供應中斷以及設備或系統故障,可能會對我們的聲譽和運營結果產生不利影響。我們的客戶依賴我們安全地存儲、及時檢索和運輸他們的關鍵樣本,這些事件可能會導致服務中斷、一個或多個關鍵存儲設施和存儲在這些設施中的客戶樣本的物理損壞、一個或多個關鍵運營設施的臨時關閉或服務的臨時中斷,每一種情況都可能對我們的聲譽和運營結果產生負面影響。我們的主要存儲設施位於印第安納州印第安納波利斯,該地區容易發生龍捲風和其他惡劣天氣事件。
如果我們的設施在運營中經歷重大中斷,我們的業務可能會受到重大損害,而未能準確估計客戶需求可能會導致庫存過剩或過時。
我們的產品製造設施數量有限,服務提供的實驗室數量有限。如果其中任何一個設施的運營因自然災害、火災、電力或其他公用事業中斷、停工、戰爭或恐怖活動或其他類似事件而中斷,我們的業務可能會受到嚴重損害,因爲我們可能無法生產和運輸產品和零部件,或及時向我們的客戶提供服務。如果中斷髮生在我們需要迅速提高能力以滿足增加的需求或加快發貨時間表之際,我們其中一個設施的任何中斷的影響可能會加劇。
此外,如果對我們的產品或服務的實際需求與預期不同,我們可能會購買比必要更多/更少的零部件或其他供應品,或者因取消、推遲或加快此類零部件或供應品的交付而產生費用。如果我們預計客戶需求並未實現而購買庫存,或者如果我們的客戶減少或推遲訂單,我們可能會產生超額庫存費用。任何或所有這些因素都可能對我們的業務、財務狀況和經營業績產生重大不利影響。
我們的業務依賴於某些關鍵信息系統,此類系統的故障或破壞可能會損害我們的業務和運營結果,並且如果未經授權訪問客戶,’的數據或我們的數據會招致重大的法律和財務風險和責任。
我們利用某些關鍵的信息技術系統和網絡,包括由第三方提供的系統和網絡,來處理、傳輸和存儲與我們的業務相關的電子信息,以及更廣泛地爲我們的業務的有效運營而使用。這些信息系統包括電信、互聯網、我們的公司內聯網、各種計算機硬件和軟件應用程序、網絡通信和電子郵件。這些信息系統可能由我們、我們的外包供應商或其他第三方(如供應商和承包商)擁有和維護。隨着數字技術的使用增加,網絡安全事件,包括蓄意攻擊和企圖未經授權訪問計算機系統和網絡,頻率和複雜性都有所增加,而且越來越難以檢測。這些威脅對我們的系統和網絡的安全以及我們的數據的機密性、可用性、可靠性、充分性和完整性構成了風險。不能保證我們將成功地預防或檢測網絡安全事件和攻擊,或成功地減輕其影響。
儘管實施了安全措施,我們的信息技術系統以及第三方提供的信息技術系統很容易受到黑客攻擊、計算機病毒、惡意軟件(包括勒索軟件)、軟件錯誤、未經授權的訪問、自然災害、恐怖主義、戰爭和電信、設備和電氣故障的損害或中斷。我們無法在關鍵時間點使用或訪問這些信息系統,或者未經授權訪問或獲取機密或專有信息或個人數據,可能會對我們的聲譽以及我們業務的及時有效運營產生不利影響。
我們已採取措施,旨在防止並在必要時發現和應對此類網絡安全事件和違反隱私和安全任務的行爲。我們預防、發現、應對和儘量減少此類風險的措施可能不會成功。雖然據我們所知,到目前爲止,我們還沒有經歷過任何重大的系統故障、事故或重大網絡安全事件,但如果發生此類事件並導致我們的運營或與我們簽約的第三方的運營中斷,可能會導致法律上的損害,以及我們的計劃和業務運營以及我們的財務狀況的實質性中斷。如果任何中斷或網絡安全事件導致我們的數據或應用程序丟失或損壞,或不適當的披露、丟失、腐敗、修改或竊取機密或專有信息或個人數據,除了招致責任外,我們的產品和服務的進一步開發可能會延遲,或者我們的競爭地位可能會受到損害。此外,此類中斷或網絡安全事件可能導致美國或外國監管機構的執法行動、監管處罰和其他法律責任,例如但不限於私人訴訟、巨額補救費用、對我們的開發計劃、業務運營和合作的中斷、管理工作的轉移以及對我們聲譽的損害,所有這些都可能損害我們的業務和運營。
We have identified a material weakness in our internal control over financial reporting which led to a conclusion that our internal control over financial reporting is not effective as of September 30, 2024. Our ability to remediate the material weakness, the discovery of additional material weaknesses, and our inability to achieve and maintain effective disclosure controls and procedures and internal control over financial reporting, could adversely affect our results of operations, our stock price and investor confidence in our company.
Pursuant to SEC rules and regulations, our management is required to report on the effectiveness of our internal control over financial reporting. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. Annually, we perform activities that include reviewing, documenting and testing our internal control over financial reporting. If we fail to maintain the adequacy of our internal control over financial reporting, we will not be able to conclude on an ongoing basis that we have effective internal control over financial reporting. Failure to achieve and maintain effective internal control over financial reporting could result in material misstatements in our financial statements and a failure to meet our reporting and financial obligations, each of which could have a material adverse effect on our financial condition and the trading price of our common stock. This could result in significant expenses to remediate any internal control deficiencies and lead to a decline in our stock price.
We identified a material weakness in our internal control over financial reporting as of September 30, 2024 as we did not design and maintain effective controls related to the review of the cash flow statement. The material weakness resulted in immaterial misstatements in our Consolidated Statements of Cash Flows for the Q2 and Q3 interim periods during fiscal 2023, for the year ended September 30, 2023, as well as the Q1, Q2, and Q3 interim periods during fiscal 2024 and in our supplemental cash flow disclosures for the year ended September 30, 2022, each interim and annual period during fiscal 2023 and the Q1, Q2 and Q3 interim periods during fiscal 2024. Additionally, the material weakness could result in material misstatements of our interim or annual consolidated statement of cash flows or supplemental cash flow disclosures that would not be prevented or detected on a timely basis.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis. Our management may be unable to conclude in future periods that our disclosure controls and procedures are effective due to the effects of various factors, which may, in part, include unremediated material weaknesses in internal control over financial reporting.
Our management has taken, and plans to take, actions to remediate the deficiency in our internal control over financial reporting and will implement new processes, procedures and controls designed to address the underlying causes associated with the material weakness. While we expect to continue to implement our remediation plans throughout the fiscal year ended September 30, 2025, we cannot be certain as to when the remediation of this material weakness will be fully completed. During the course of completing our remedial actions, we may identify areas requiring improvement and may be required to design additional enhanced processes and controls to address issues identified through this process. In addition, there can be no assurance that such remediation efforts will be successful, that our disclosure controls and procedures or internal control over financial reporting will be effective as a result of these efforts or that any such future deficiencies identified may not be material weaknesses that would be required to be reported in future periods.
If we fail to remediate this material weakness or otherwise not maintain effective disclosure controls and procedures or internal control over financial reporting, we may not be able to rely on the integrity of our financial results or otherwise provide reliable financial statements, which could adversely affect our business decisions, result in inaccurate or late reporting of our financial results, as well as delays or the inability to meet our reporting obligations or to comply with SEC rules and regulations. Any of these could result in delisting actions by the Nasdaq Stock Market, investigation and sanctions by regulatory authorities, stockholder investigations and lawsuits, and could adversely affect our business, results of operations, ability to obtain financing and the trading price of our common stock.
Our goodwill and intangible assets may become impaired.
As of September 30, 2024, we had $691.4 million of goodwill and $248.0 million in net intangible assets as a result of our acquisitions. We periodically review our goodwill and the estimated useful lives of our identifiable intangible assets, taking into consideration any events or circumstances that might result in either a diminished fair value, or for intangible assets, a revised useful life. These events and circumstances include significant changes in the business climate, legal factors, operating performance indicators, advances in technology and competition. Any impairment or revised useful life could have a material and adverse effect on our financial position and results of operations and could harm the trading price of our common stock.
In the event the performance of any of our reporting units does not meet management expectations in the future, we experience a prolonged macroeconomic or market downturn, or there are other negative revisions to key assumptions used in the analyses used to estimate fair value, we may be required to perform an impairment analysis which could result in an impairment charge. As of October 1, 2023, we reorganized the business under three operating segments, and as a result, reallocated goodwill to the newly defined reporting units. Subsequent to this reallocation, during the second quarter of fiscal year 2024, as part of our routine long-term planning process, we assessed several events and circumstances that could affect the significant inputs used to determine the fair value of our reporting units, including updates to forecasted cash flows, the impact of our cost saving plans and planned transformation initiatives and the overall change in the economic climate since our last impairment assessment in October 2023. We concluded it was more likely than not the fair value of the B Medical Systems segment was less than its carrying amount resulting from the reduction in our anticipated revenue growth rates for the current and subsequent years as compared to prior projections. As a result, we completed a quantitative goodwill impairment test for our reporting units in accordance with Accounting Standards Codification 350, Intangibles – Goodwill as of March 31, 2024. We recorded a non-cash impairment charge of $111.3 million within "Impairment of goodwill and intangible assets" in our Condensed Consolidated Statements of Operations during the three months ended March 31, 2024. For further details refer to Note 8, Goodwill and Intangible Assets to our Consolidated Financial Statements included under Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K. Any additional impairment charges could negatively impact our business and stock price as set forth above.
Changes in tax rates or tax regulation could affect results of operations.
As a global company, we are subject to taxation in the United States and various other countries. Significant judgment is required to determine and estimate worldwide tax liabilities. Our future annual and quarterly effective tax rates could be affected by numerous factors, including changes in the following: applicable tax laws; composition of pre-tax income in countries with differing tax rates; and/or establishment of a valuation allowance against deferred tax assets based on the assessment of their realizability prior to expiration. Changes in applicable tax laws could significantly impact the estimates of our tax assets and liabilities, as well as expectations of future effective tax rates. Changes in tax laws could also negatively impact our ability to move our cash balances between the jurisdictions in which we operate. In addition, we are subject to regular examination by the U.S. Internal Revenue Service and state, local and foreign tax authorities. We regularly assess the likelihood of favorable or unfavorable outcomes resulting from these examinations to determine the adequacy of our expense for income taxes. Although we believe our tax estimates are reasonable, there can be no assurance that any final determination will not be materially different from the treatment reflected in our historical income tax (benefits) expenses and accruals, which could materially and adversely affect our financial condition and results of operations.
International trade disputes could result in additional or increased tariffs, export controls or other trade restrictions that may have a material impact on our business.
We sell a significant number of products outside the United States, including in China and Africa. Based on the complex relationships among these countries and the United States, there is inherent risk that political, diplomatic and national security influences might lead to trade disputes, impacts and/or disruptions. The United States and other countries have imposed and may continue to impose trade restrictions and have also levied tariffs and taxes on certain goods. Increases in tariffs, additional taxes or other trade restrictions and retaliatory measures may increasingly impact customer demand and customer investment in manufacturing equipment, increase our manufacturing costs, decrease margins, reduce the competitiveness of our products, or inhibit our ability to sell products or purchase necessary equipment and supplies, which could have a material adverse effect on our business, results of operations, or financial condition.
In addition, a portion of the manufacturing for our products and providing services takes place in China through third-party manufacturers and service providers. The BIOSECURE Act that was recently passed by the U.S. House of Representatives is aimed at discouraging federal contracting with certain Chinese biotechnology companies for biotechnology equipment or services. If the BIOSECURE Act becomes law, its implementation has the potential to impact supply of our products and services. Additionally, if following the enactment and implementation of the BIOSECURE ACT we are required to change manufacturers or service providers for any reason, we will be required to verify that the new manufacturer or provider maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. We anticipate that the complexity of our processes may impact the amount of time it may take to secure a replacement manufacturer or provider and such delays could negatively affect our ability to develop and sell products and services, which could have a material adverse effect on our business, results of operations, or financial condition.
We are subject to numerous governmental regulations.
我們受聯邦、州、地方和外國法規的約束,包括環境法規、與我們產品的設計和運營相關的法規以及與我們提供的某些服務相關的控制系統和法規,包括上文第1項「業務-環境事項和治理法規」中描述的那些法規。當我們試圖確保我們的產品符合安全和排放標準時,我們可能會產生巨大的成本,其中許多標準在使用我們產品的州和國家/地區有所不同,並且我們的多重組學業務的GLP監管服務是在經過認證和認證的實驗室進行的。在過去,我們投入了大量資源來重新設計我們的產品,並建立和維護我們的實驗室以符合這些法規。爲了遵守未來的法規、指令和標準,我們可能需要修改或重新設計一些產品,改變我們的服務產品,進行資本支出,或者產生巨額成本。如果我們不遵守當前或未來的法規、指令和標準:
| ● |
我們可能會被罰款; |
| ● |
我們的生產或發貨可能會暫停;和 |
| ● |
我們可能會被禁止在指定市場提供特定產品或服務。 |
任何這些事件都可能對我們的業務、財務狀況和經營業績產生重大不利影響。
我們的實際或感知到的未遵守數據保護法律和法規可能會導致政府執法行動、私人訴訟和/或不利宣傳,並可能對我們的 公事。
我們受到國內和國際數據保護法律法規的約束,這些法律法規涉及隱私和數據安全,並可能影響我們個人信息的收集、使用、存儲和傳輸。數據保護的立法和監管格局繼續發展,近年來,人們越來越關注隱私和數據安全問題,這可能會影響我們的業務。在美國,許多聯邦和州法律法規,包括州數據泄露通知法、州健康信息隱私法以及聯邦和州消費者保護法,管理着與健康相關的個人信息和其他個人信息的收集、使用、披露和保護。如果不遵守數據保護法律和法規(如適用),可能會導致政府執法行動,這可能包括民事或刑事處罰、私人訴訟和/或負面宣傳,並可能對我們的經營業績和業務產生負面影響。例如,加利福尼亞州已經頒佈了加州消費者隱私法案,該法案於2020年1月生效。CCPA賦予加州居民更大的權利,可以訪問和要求刪除他們的個人信息,選擇不共享某些個人信息,並獲得有關他們的個人信息如何使用的詳細信息。CCPA規定了對違規行爲的民事處罰,以及對可能增加數據泄露訴訟的數據泄露的私人訴權。儘管CCPA包括對某些類別的健康信息的豁免,但該法律可能會增加我們在收集有關加州居民的其他個人信息方面的合規成本和潛在責任。此外,2020年,加州選民通過了加州隱私權法案,或CPRA,該法案於2023年1月1日全面生效。《全面和平協議》對《全面和平協議》進行了重大修訂,有可能導致進一步的不確定性、爲遵守《全面和平協議》而付出的額外成本和開支,以及不遵守《全面和平協議》的額外傷害和責任。除其他外,CPRA建立了一個新的監管機構-加州隱私保護局,該機構的任務是根據CPRA制定新的法規,並擴大了執法權力。除了加利福尼亞州,美國還有更多的州正在制定類似的法律,這增加了合規的複雜性,增加了未能遵守的風險。2023年,弗吉尼亞州、科羅拉多州、康涅狄格州和猶他州的全面隱私法都生效了,蒙大拿州、俄勒岡州和德克薩斯州的法律也在2024年生效。此外,美國其他州的法律將在2024年後生效,美國其他州也在考慮提案,所有這些都可能增加我們的監管合規成本和風險、面臨監管執法行動的風險以及其他責任。
其他許多國家也有或正在制定管理個人信息收集、使用和傳輸的法律。例如,歐洲議會和歐洲聯盟理事會通過了一個全面的一般數據隱私框架,稱爲一般數據保護條例(GDPR),該框架於2018年5月生效,對歐盟境內(包括歐盟以外的公司)收集和使用個人數據進行監管。GDPR的範圍很廣,對與個人數據有關的個人的同意、提供給個人的信息、個人數據的安全和保密、數據泄露通知以及在處理個人數據時使用第三方處理器等方面提出了幾項要求。GDPR還對將個人數據從歐盟轉移到美國實施了嚴格的規則,加強了執法權力,並對違反GDPR的行爲施加了巨額處罰,包括可能對侵權者處以最高2,000歐元萬或侵權者全球年收入4%的罰款,以數額較大者爲準。GDPR還授予數據主體和消費者協會向監管機構投訴、尋求司法補救和獲得因違反GDPR而造成的損害賠償的私人權利。遵守GDPR一直是並將繼續是一個嚴格和耗時的過程,已經增加,並將繼續增加我們的業務成本或要求我們改變我們的業務做法,儘管我們做出了這些努力,但我們可能面臨與任何歐洲活動相關的罰款和處罰、訴訟和聲譽損害的風險,這可能會對我們的業務、前景、財務狀況和運營結果產生不利影響。
此外,在英國退出歐盟(即,英國脫歐),以及英國脫歐過渡期(於2020年12月31日結束)到期後,GDPR已在英國實施(稱爲英國GDPR)。英國GDPR與2018年英國數據保護法並列,該法將歐盟GDPR中的某些克減到英國法律中。根據英國GDPR,非在英國成立但處理與向英國個人提供商品或服務相關的個人數據或監控其行爲的公司將受到英國GDPR的約束-其要求如下(目前)基本上與歐盟GDPR下的條款一致,因此,可能會導致類似的合規和運營成本,並可能處以高達1750萬英鎊或全球營業額的4%的罰款。
適用的數據隱私和數據保護法律可能會相互衝突,通過遵守一個司法管轄區的法律或法規,我們可能會發現我們違反了另一個司法管轄區的法律或法規。儘管我們做出了努力,但我們過去可能沒有完全遵守,將來也可能沒有完全遵守。這可能需要我們承擔大量費用,這可能會嚴重影響我們的業務。不遵守數據保護法可能會讓我們面臨數據保護當局或其他監管機構採取執法行動的風險、某些司法管轄區的私人訴訟權,以及如果我們被發現不合規,可能會面臨重大處罰的風險。此外,與數據安全事件和隱私侵犯相關的政府調查數量不斷增加,政府調查通常需要大量資源併產生負面宣傳,這可能會損害我們的業務和聲譽。
與衝突礦物相關的法規和客戶要求可能會對我們產生不利影響。
《多德-弗蘭克華爾街改革和消費者保護法》對我們的產品零部件中使用從剛果民主共和國和鄰近國家開採的「衝突礦物」實施了披露要求,無論我們的產品零部件是由我們還是第三方製造的。這一要求可能會影響我們產品中使用的零部件製造中使用的礦物的定價、採購和可用性。此外,遵守披露要求和客戶要求還會產生額外成本,例如與我們確定產品中使用的任何衝突礦物來源以及準備並向SEC提交所需報告的盡職調查相關的成本。我們可能會在滿足客戶方面遇到困難,他們可能要求我們產品的所有組件都經過認證,不含衝突礦物質和/或不含許多其他危險材料。
與我們的知識產權相關的風險
我們未能保護知識產權可能會對我們未來的運營產生不利影響。
我們的競爭能力受我們保護知識產權的能力的影響很大。我們依靠專利、商業祕密法律、保密協議和程序、版權、商標和許可協議來保護我們的技術。現有的商業祕密、商標法和版權法只能提供有限的保護。我們的成功在一定程度上取決於我們在美國和其他國家爲我們的產品和服務獲得和實施專利保護的能力。我們擁有大量的美國和外國專利,我們打算根據需要提交更多的專利申請,涵蓋我們的產品、服務和技術。我們擁有或授權給我們的任何已頒發的專利可能會受到挑戰、無效或規避,這些專利下的權利可能不會爲我們提供競爭優勢。此外,我們產品和服務的開發、製造、提供或銷售所在的一些國家/地區的法律可能不能完全保護我們的產品和服務。由於生命科學和相關過程設備行業的快速技術變化,我們認爲,在建立和保持競爭優勢方面,改進現有技術、依賴商業祕密、非專利專有技術以及開發新產品或服務可能與專利保護同等重要。爲了保護商業祕密和專有技術,我們的政策是要求所有技術和管理人員簽訂保密協議。
我們無法保證我們爲保護知識產權而採取的措施足以防止我們的技術被挪用。其他公司可以在不侵犯我們知識產權的情況下獨立開發類似或更好的技術。未來,可能有必要進行訴訟或類似活動來執行我們的知識產權、保護我們的商業祕密或確定他人(包括我們的客戶)專有權的有效性和範圍。這可能需要我們承擔巨額費用,並轉移我們管理和技術人員的精力和注意力從我們的業務運營上。
隨着時間的推移,我們的專利到期可能會導致競爭加劇和我們的收入下降。
我們的主要競爭優勢之一是我們的技術,我們依賴我們的專利權和其他知識產權來維持我們的競爭地位。我們的專利從2024年開始在不同日期開始到期,並將在此後持續到2042年,這可能會導致競爭加劇以及產品和服務收入下降。
我們可能會受到侵犯第三方知識產權的指控,或要求我們許可第三方技術,這可能會導致巨額費用並阻止我們使用我們的技術。
在我們開展業務的行業中,存在有關專利和其他知識產權的大量訴訟。我們過去曾被告知,並且將來可能被告知,我們可能侵犯了第三方擁有的知識產權。我們不能保證第三方的侵權索賠或我們產品和服務的客戶或最終用戶因侵權索賠而提出的其他賠償索賠將來不會提出,或者此類索賠(無論是否被證明屬實)不會對我們的業務、財務狀況和運營結果產生重大不利影響。
我們無法預測我們可能需要在多大程度上尋求許可或改變我們的產品或服務,以便它們不再侵犯他人的權利。我們也無法保證許可證的可用,或者我們可能需要獲得的任何許可證的條款是合理的。同樣,爲避免侵犯他人權利而改變我們的產品、服務或流程可能成本高昂或不切實際,並且可能會降低我們產品和服務的價值。如果我們被判侵權,我們可能會被要求支付巨額損害賠償,法院可能會發布命令,阻止我們銷售一種或多種產品或提供某些服務。此外,即使我們獲勝,此類訴訟帶來的成本和管理層注意力的轉移也可能是巨大的。任何這些事件都可能給我們帶來巨額費用,並可能對我們的業務和前景造成重大損害。
與依賴第三方相關的風險
如果一個或多個關鍵供應商未能持續交付可接受的成本和質量的關鍵零部件,我們的業務可能會受到重大損害。
目前,我們根據需要從衆多供應商處採購訂單獲取許多關鍵零部件。在某些情況下,我們產品製造中使用的關鍵零部件和材料只有單一供應來源。此外,我們的一部分供應來自亞洲,包括中國,而且我們之前並不總是有與這些供應商打交道的歷史。我們無法獲得所需數量或可接受的成本和質量以及必要的供應連續性的零部件或材料,可能會導致向客戶發貨的產品延遲或減少。此外,如果供應商或子供應商因任何原因(包括自然災害或健康相關威脅)而停產或延誤,這可能會導致我們向客戶的產品發貨延遲或減少。任何這些意外情況都可能導致我們失去客戶,導致收入延遲或損失,並對我們的業務造成重大損害。
Our business could be adversely affected by a decline in the availability of raw materials.
We are dependent on the availability of certain key raw materials and natural resources used in our products and various manufacturing processes, and we rely on third parties to supply us with these materials in a cost-effective and timely manner. Our access to raw materials may be adversely affected if our suppliers’ operations were disrupted as a result of limited or delayed access to key raw materials and natural resources which may result in increased cost of these items.
Our external service providers may fail to perform as we expect or may suffer cybersecurity breaches.
Our external service providers have played and will continue to play a key role in many of our transactional and administrative functions, such as information technology and facilities management. Many of these service providers, including certain hosted software applications that we use for the storage and processing of confidential, proprietary, or personal information, employ various processing and storage technologies, including cloud computing technology. These providers’ information technology systems may be susceptible to cybersecurity incidents and breaches, attacks by hackers, or other incidents, including those due to employee error, malfeasance, or other disruptions, which are outside of our control. Although we attempt to select reputable providers, perform diligence on such providers, and enter into written contracts, it is possible that one or more of these providers could fail to perform or adequately protect our data from cybersecurity incidents as we expect, and any such failure could have an adverse impact on our business. Any such incident could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, regulatory penalties, disrupt our operations, damage our reputation, and cause a loss of confidence in us and our ability to conduct our business and our competitive advantage, which could adversely affect our reputation.
Risks Relating to Our Customers
Customers generally do not make long term commitments to purchase our products and our customers may cease purchasing our products at any time.
Sales of our products are often made pursuant to individual purchase orders and not under long-term commitments and contracts. Our customers frequently do not provide any assurance of minimum or future sales and are not prohibited from purchasing products from our competitors at any time. Accordingly, we are exposed to competitive pricing pressures on each order.
We may face claims for liability related to damages of customer materials attributed to the failure of our products or services, exposing us to significant financial or reputational harm.
Our automated cold storage systems are used in the handling, movement and storage of biological and chemical samples. We also provide sample storage services to customers where we store their biological and chemical samples or perform genomic services at our facilities. In any case, in addition to product warranty claims, inaccurate or faulty testing services or damage to our customers’ materials attributed to a failure of our products or services could lead to additional claims for damages made by our customers and could also harm our relationship with our customers and damage our reputation, resulting in material harm to our business.
Risks Relating to Owning Our Securities
Our stock price is volatile.
The market price of our common stock has fluctuated widely. From the beginning of fiscal year 2023 through the end of fiscal year 2024, our stock price fluctuated between a high of $67.51 per share and a low of $36.45 per share. Consequently, the current market price of our common stock may not be indicative of future market prices, and we may be unable to sustain or increase the value of an investment in our common stock. Factors affecting our stock price may include:
| ● |
variations in operating results from quarter-to-quarter and year-to-year; |
| ● |
changes in earnings estimates by analysts or our failure to meet analysts’ expectations; |
| ● |
changes in the market price per share of our public company customers and competitors; |
| ● |
the completion of repurchases of our common stock under our 2022 share repurchase authorization; |
| ● |
the timing and amount of any new repurchases of our common stock; |
| ● |
market conditions in the life sciences sample management and genomic services and other industries into which we sell products and services; |
| ● |
global economic conditions; |
| ● |
political changes, hostilities, public health threats, or natural disasters such as hurricanes and floods; |
| ● |
low trading volume of our common stock; |
| ● |
the number of firms making a market in our common stock; and |
| ● |
actions of activist stockholders and our response(s) thereto. |
In addition, the stock market has in the past experienced significant price and volume fluctuations. These fluctuations have particularly affected the market prices of the securities of life sciences companies like ours. These market fluctuations could adversely affect the market price of our common stock.
Our business and operations could be negatively affected by securities litigation or stockholder activism, which could impact the trading price and volatility of our common stock and may constrain capital deployment opportunities and adversely impact our ability to expand our business.
Our business and operations could be negatively affected if we become subject to any securities litigation or from continued stockholder activism, which could cause us to incur significant expenses, hinder the execution of our business and growth strategy, constrain our capital deployment opportunities, and impact the price of our common stock. Stockholder activism, which can take many forms or arise in a variety of situations, has been increasing recently. Volatility in the price of our common stock, our cash balance, our financial performance or other reasons may cause us to become the target of securities litigation or continue to be the target of stockholder activism.
We have been and may continue to be subject to stockholder activism, including relating to the actions of Politan Capital Management LP, or Politan, described in the Schedule 13D that it initially filed with the SEC on September 14, 2023, as amended, and may be subject to continued and other stockholder activism in the future. For example, on November 1, 2024, we entered into a Cooperation Agreement with Politan pursuant to which we agreed, among other things: (a) to increase the size of the Board of Directors by three (3) directors and appoint Quentin Koffey, the Managing Partner and Chief Investment Officer of Politan, to our Board of Directors; (b) to establish a new Value Creation Committee of the Board of Directors, or the Committee; (c) to appoint Mr. Koffey, William Cornog, Alan Malus, Martin Madaus and John Marotta to the Committee; (d) to appoint Mr. Koffey to the Human Resources and Compensation Committee of the Board of Directors; (e) to nominate the members of the Committee for election to the Board of Directors at our 2025 Annual Meeting of Stockholders; and (f) that two directors serving on the Board of Directors immediately prior to the execution of the Cooperation Agreement would not stand for re-election to the Board of Directors at our 2025 Annual Meeting of Stockholders. Perceived uncertainties as to our future direction as a result of these actions or any future stockholder activism or further changes to the composition of our Board of Directors or management may lead to the perception of a change in the direction of our business, instability or lack of continuity, any of which could negatively impact our stock price and results of operations.
Securities litigation and stockholder activism, including potential proxy contests, could result in substantial costs and divert management’s and our Board of Director’s attention and resources from our business. Additionally, such securities litigation and stockholder activism could give rise to perceived uncertainties as to our future, adversely affect our relationships with service providers and make it more difficult to attract and retain qualified personnel. Also, we have and may be required to incur significant legal fees and other expenses related to any securities litigation and activist stockholder matters. Further, the price of our common stock could be subject to significant fluctuation or otherwise be adversely affected by the events, risks and uncertainties of any securities litigation and stockholder activism. In addition, stockholder activism may constrain our capital deployment opportunities and may limit the types of investments that are available to us.
Provisions in our charter documents and Delaware law may delay or prevent an acquisition of us, which could decrease the value of your shares.
Our restated certificate of incorporation and by-laws and Delaware law contain provisions that could make it harder for a third party to acquire us without the consent of our Board of Directors. These provisions include limitations on actions by our stockholders by written consent, the inability of stockholders to call special meetings, requiring advance notice in accordance with our by-laws for stockholder proposals that can only be acted upon at annual stockholder meetings and nominations to our Board of Directors, limiting the approval of changes in the number of directors to our Board of Directors or by a super majority vote of our stockholders and the potential for super majority votes of our stockholders in certain other circumstances. In addition, as discussed below, our Board of Directors has the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer.
Our restated certificate of incorporation makes us subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits publicly held Delaware corporations to which it applies from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Our restated certificate of incorporation also contains anti-greenmail provisions which prohibit us from repurchasing our common stock from certain related persons unless specific conditions are satisfied. These provisions could discourage others from bidding for our shares of common stock and could, as a result, reduce the likelihood of an increase in the price of our common stock that would otherwise occur if a bidder sought to buy our common stock.
Although we believe these provisions provide for an opportunity to receive a higher bid by requiring potential acquirers to negotiate with our Board of Directors, these provisions apply even if the offer may be considered beneficial by stockholders. If a change of control or change in management is delayed or prevented by these provisions, the market price of our common stock could decline.
Our restated certificate of incorporation authorizes the issuance of shares of blank check preferred stock.
Our restated certificate of incorporation provides that our Board of Directors is authorized to designate and issue from time to time, without further stockholder approval, up to 1,000,000 shares of preferred stock in one or more series and to fix and designate the rights, preferences, privileges and restrictions of the preferred stock, including dividend rights, conversion rights, voting rights, redemption rights and terms of redemption and liquidation preferences. Such shares of preferred stock could have preferences over our common stock with respect to dividends and liquidation rights. Our designation and issuance of preferred stock, including in connection with the adoption of a stockholders rights plan, or “poison pill,” may have the effect of delaying or preventing a change in control. Our issuance of preferred stock could decrease the amount of earnings and assets available for distribution to the holders of common stock or could adversely affect the rights and powers, including voting rights, of the holders of common stock. The issuance of preferred stock could have the effect of decreasing the market price of our common stock.
Our by-laws designate the state courts in the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could discourage lawsuits against the company and our directors, officers and employees.
Our by-laws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery of the State of Delaware does not have jurisdiction, the federal district court for the District of Delaware) will be the sole and exclusive forum for the following types of proceedings:
| ● |
any derivative action or proceeding brought on our behalf; |
| ● |
any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, employees or stockholders to our company or our stockholders; |
| ● |
any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our by-laws; or |
| ● |
any action asserting a claim governed by the internal affairs doctrine of the law of the State of Delaware. |
These choice of forum provisions will not apply to causes of action arising under the Securities Act or the Exchange Act or any other claim for which federal courts have exclusive jurisdiction. Furthermore, our by-laws provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any claims under the Securities Act.
These exclusive forum provisions may limit the ability of our stockholders to bring a claim in a judicial forum that such stockholders find favorable for disputes with us or our directors, officers or employees, which may discourage such lawsuits against us and our directors, officers and employees. Alternatively, if a court were to find the choice of forum provisions contained in our by-laws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could materially adversely affect our business, financial condition and operating results.
Item 1B. Unresolved Staff Comments
None.
Risk Management
We have implemented a cybersecurity risk management program intended to protect the confidentiality, integrity, and availability of our critical systems and information. Our cybersecurity risk management program is an element of and is integrated into our overall enterprise risk management program, and is a key component of our annual organizational risk assessment. Our cybersecurity risk management program is based in part on, and incorporates elements of, the National Institute of Standards and Technology (NIST) Cybersecurity Framework and International Organization for Standardization 27001 (ISO 27001) Framework. In general, we seek to address cybersecurity risks through a comprehensive, cross-functional approach that is focused on preserving the confidentiality, security and availability of the information that we collect and store by identifying, preventing and mitigating cybersecurity threats and effectively responding to cybersecurity incidents when they occur.
Our cybersecurity risk management program utilizes a variety of technical and process controls that are designed to identify, protect against, detect, respond to, and recover from cybersecurity threats, including:
| ● |
risk assessments designed to help identify material cybersecurity risks to our critical systems, information, products, services, and our broader enterprise information technology (“IT”) environment; |
| ● |
a security team that is principally responsible for managing (1) our cybersecurity risk assessment processes, (2) our security controls and policies, and (3) our response to cybersecurity incidents; |
| ● |
the use of external service providers, where appropriate, to assess, test, or otherwise assist with aspects of our security controls; |
| ● |
cybersecurity awareness training for our employees, incident response personnel, and senior management; |
| ● |
assessment of material cybersecurity risks posed by third-party service providers, including risks to employee, customer and financial information; |
| ● |
a cybersecurity incident response protocol that includes procedures for responding to cybersecurity incidents; and |
| ● |
business continuity plans. |
As part of the above processes, we engage, as necessary, consultants and other third parties, to review our cybersecurity incidents if material to help quantify the impact and identify areas for continued focus, improvement, and compliance.
Our processes also address cybersecurity threat risks associated with our use of third-party service providers, including our suppliers and manufacturers or who have access to confidential, proprietary, personal, or employee data, or to our systems. In addition, cybersecurity considerations affect the selection and oversight of our third-party service providers. We perform diligence on third parties that have access to our systems, data or facilities that house such systems or data, and continually monitor cybersecurity threat risks identified through such diligence. Additionally, we generally require those third parties that could introduce significant cybersecurity risk to us to agree by contract to manage their cybersecurity risks in specified ways, and to agree to be subject to cybersecurity audits or audits for System and Organization Controls (SOC) compliance.
We have been, and expect to continue to be, subject to cybersecurity risks and incidents related to our business. We have not experienced any material cybersecurity incidents during the last fiscal year. For more information about the cybersecurity risks we face, see Item 1A – Risk Factors of this Annual Report on Form 10-K.
Governance
Our Board considers cybersecurity risk as part of its enterprise risk management oversight function. The Board delegates oversight of the cybersecurity risk management program to the Audit Committee. This oversight includes periodic reports from management concerning cybersecurity related risks. The management of the program is the responsibility of our Risk Management Committee, comprised of our Chief Financial Officer, Chief Digital & Information Officer, Chief Accounting Officer and General Counsel. Our Chief Digital & Information Officer, who has over 30 years of extensive work experience in the field of technology and cybersecurity, leads our team of cybersecurity professionals and provides the Risk Management Committee with periodic reports on our cybersecurity risks and any material cybersecurity incidents. Our team of cybersecurity professionals monitors the prevention, mitigation, detection, and remediation of cybersecurity incidents through the cybersecurity risk management and processes described above, including the operation of our incident response plan. The Risk Management Committee provides updates to the Audit Committee on our cybersecurity risk management program as appropriate, including updates on (1) any critical cybersecurity risks; (2) ongoing cybersecurity initiatives and strategies; (3) applicable regulatory requirements; and (4) industry standards. The Risk Management Committee also notifies the Board of any significant and/or material cybersecurity incidents (suspected or actual) and provides updates on the incidents as well as cybersecurity risk mitigation activities as appropriate.
Our corporate headquarters are currently located in Burlington, Massachusetts. We maintained the following principal facilities as of September 30, 2024:
| Square Footage |
Ownership Status/ |
|||||||||
| Location |
Functions |
Segment |
(Approx.) |
Lease Expiration |
||||||
| Suzhou, China |
Laboratory & office |
Multiomics |
240,000 | Owned |
||||||
| Hosingen, Luxembourg |
B Medical headquarters & manufacturing |
B Medical Systems |
228,000 | Owned |
||||||
| Indianapolis, Indiana |
Sample storage, sales & support |
Sample Management Solutions |
116,700 | September 2043 |
||||||
| Billerica, Massachusetts |
Sample storage, R&D and office |
Sample Management Solutions |
39,900 | October 2033 |
||||||
| South Plainfield, New Jersey |
Laboratory and office |
Multiomics |
73,300 | January 2030 |
||||||
| Plainfield, Indiana |
Manufacturing, R&D and sales & support |
Sample Management Solutions |
67,900 | August 2042 |
||||||
| Springfield, Missouri |
Manufacturing, R&D and sales & support |
Sample Management Solutions |
50,100 | December 2028 |
||||||
| Manchester, United Kingdom |
Manufacturing and office |
Sample Management Solutions |
44,700 | December 2029 |
||||||
| Burlington, Massachusetts |
Corporate headquarters, training, R&D and sales & support |
All |
26,200 | October 2025 |
||||||
In addition to the principal facilities listed above, we maintain additional laboratories, biorepositories, and sales and support offices throughout North America, Europe, and Asia. The Company believes that its facilities are in good physical condition, are suitable and adequate for the operations conducted at those facilities and are generally fully utilized and operating at normal capacity.
We are subject to various legal proceedings, both asserted and unasserted, that arise in the ordinary course of business. We cannot predict the ultimate outcome of such legal proceedings or in certain instances provide reasonable ranges of potential losses. However, as of the date of this Annual Report on Form 10-K, we believe that none of these claims will have a material adverse effect on our consolidated financial condition or results of operations. In the event of unexpected subsequent developments and given the inherent unpredictability of these legal proceedings, there can be no assurance that our assessment of any claim will reflect the ultimate outcome and an adverse outcome in certain matters could, from time-to-time, have a material adverse effect on our consolidated financial condition or results of operations in particular quarterly or annual periods.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded on the Nasdaq Stock Market LLC, or Nasdaq under the symbol “AZTA.”
Number of Holders
As of November 19, 2024, there were 455 holders of record of our common stock.
Dividend Policy
Dividends are declared at the discretion of our Board of Directors and depend on actual cash flow from operations, our financial condition, capital requirements and any other factors our Board of Directors may consider relevant.
Since the completion of the sale of the semiconductor automation business on February 1, 2022, we have not paid a quarterly dividend and do not have plans to pay any dividends at this time.
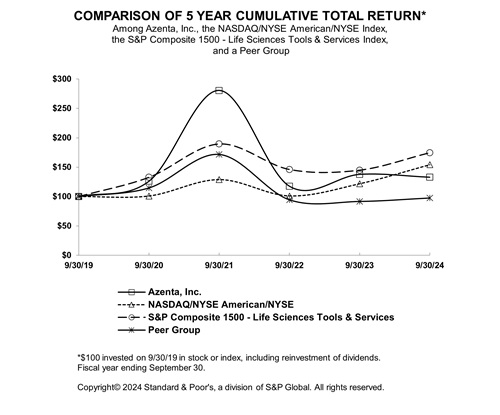
Comparative Stock Performance
The following graph compares the cumulative total shareholder return (assuming reinvestment of dividends) from investing $100 on September 30, 2019, and plotted at the last trading day of each of the fiscal years ended September 30, 2020, 2021, 2022, 2023 and 2024, in each of (i) our Common Stock; (ii) the Nasdaq/NYSE American/NYSE Index of companies; (iii) S&P 1500 Life Sciences Tools & Services Industry Index; and (iv) a peer group.
The peer group is comprised of Angiodynamics Inc, Caredx Inc, Certara Inc, Haemonetics Corp, Icu Medical Inc, Integra Lifesciences Holdings Corp, Maravai Lifesciences Holdings Inc, Medpace Holdings Inc, Neogenomics Inc, Orasure Technologies Inc, Repligen Corp, Sotera Health Co, and Varex Imaging Corp. This is the same peer group that we compared our total shareholder return to for the fiscal year ended September 30, 2023. We have decided to compare our total shareholder return to that of the S&P 1500 Life Sciences Tools & Services Industry Index as opposed to that of the peer group used for the fiscal year ended September 30, 2023 to align our “Comparative Stock Performance” disclosures with that of the same line-of-business index to which we compare our executive performance in our “Pay Versus Performance” disclosures in our proxy statements for the annual meeting of our stockholders.

| 9/30/2019 |
9/30/2020 |
9/30/2021 |
9/30/2022 |
9/30/2023 |
9/30/2024 |
|||||||||||||
| Azenta, Inc. |
$ | 100.00 | $ | 126.14 | $ | 280.45 | $ | 117.55 | $ | 137.65 | $ | 132.85 | ||||||
| NASDAQ/NYSE American/NYSE |
100.00 | 100.86 | 128.93 | 100.95 | 121.74 | 154.22 | ||||||||||||
| S&P Composite 1500 - Life Sciences Tools & Services |
100.00 | 132.71 | 189.49 | 146.05 | 144.81 | 174.57 | ||||||||||||
| Peer Group |
100.00 | 114.79 | 171.86 | 94.45 | 91.66 | 97.53 | ||||||||||||
The information included under the heading “Comparative Stock Performance” in this Item 5 of this Annual Report on Form 10-K shall not be deemed to be “soliciting material” or subject to Regulation 14A, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act.
Issuer Purchases of Equity Securities
The following provides information about repurchases of our common stock during the three months ended September 30, 2024:
| Total |
Total Number of Shares |
Approximate Dollar Value |
||||||||||||||||
| Number of |
Average |
Purchased As Part of |
Of Shares That May |
|||||||||||||||
| Shares |
Price Paid |
Publicly Announced |
Yet Be Purchased |
|||||||||||||||
| Purchased |
Per Share |
Plans or Programs |
(in millions) |
|||||||||||||||
| Period of Repurchase |
Repurchase Program |
(#) |
($) |
(#) |
($) |
|||||||||||||
| July 1 - 31, 2024 |
Open market repurchase |
1,549,506 | $ | 54.12 | 26,666,626 | $ | 165 | |||||||||||
| August 1 - 31, 2024 |
Open market repurchase |
1,693,619 | 51.89 | 28,360,245 | 77 | |||||||||||||
| September 1 - 30, 2024 |
Open market repurchase |
1,612,743 | 47.79 | 29,972,988 | — | |||||||||||||
| Total |
4,855,868 | $ | 51.24 | |||||||||||||||
On November 4, 2022, our Board of Directors approved a share repurchase authorization for the repurchase of up to $1.5 billion of our common stock (the “2022 Repurchase Authorization”). In November 2022, as part of the 2022 Repurchase Authorization, we entered into an accelerated share repurchase agreement (the “ASR Agreement”) with JPMorgan Chase Bank, National Association for the repurchase of $500 million of our common stock which terminated and settled in April 2023. Following the termination of the ASR Agreement, we entered other arrangements under the 2022 Repurchase Authorization in order to repurchase the remaining $1.0 billion shares of common stock authorized for repurchase through open market purchases, intended to qualify under Rule 10b5-1 under the Exchange Act. During the three months ended September 30, 2024, we repurchased 4.9 million shares of common stock for approximately $248.8 million (excluding fees, commissions, and excise tax) through open market repurchases under these other arrangements which completed our share buyback under the 2022 Repurchase Authorization. As of September 30, 2024, we have no remaining authorization for share repurchases.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our Consolidated Financial Statements and related notes appearing elsewhere in this Annual Report on Form 10-K. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those discussed below and in the forward-looking statements. Factors that could cause or contribute to these differences include, without limitation, those discussed in “Information Related to Forward-Looking Statements” and Part I, Item 1A, “Risk Factors” included above in this Annual Report on Form 10-K.
This Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A, describes principal factors affecting the results of our operations, financial condition and liquidity, as well as our critical accounting policies and estimates that require significant judgment and thus have the most significant potential impact on our Consolidated Financial Statements included elsewhere in this Annual Report on Form 10-K. All dollar amounts in the below MD&A are presented in U.S. dollars, unless otherwise noted or the context otherwise provides.
Unless noted otherwise, this MD&A relates solely to our continuing operations and does not reflect the operations of the semiconductor automation business which we sold to Thomas H. Lee Partners, L.P. for $2.9 billion in cash on February 1, 2022, which is reflected as discontinued operations in our Consolidated Financial Statements.
Our MD&A is organized as follows:
| ● |
Overview. This section provides a general description of our business and operating segments as well as a brief discussion and overall analysis of our business and financial performance, including key developments affecting us during the fiscal years ended September 30, 2024 and 2023. |
| ● |
Critical Accounting Policies and Estimates. This section discusses accounting policies and estimates that require us to exercise subjective or complex judgments in their application. We believe these accounting policies and estimates are important to understanding the assumptions and judgments incorporated in our reported financial results. |
| ● |
Results of Operations. This section provides an analysis of our financial results for the fiscal year ended September 30, 2024 compared to the fiscal year ended September 30, 2023. |
| ● |
Liquidity and Capital Resources. This section provides an analysis of our liquidity and changes in cash flows, as well as a discussion of contractual commitments. |
OVERVIEW
General
We are a leading global provider of biological and chemical compound sample exploration and management solutions for the life sciences industry. We entered the life sciences market in 2011, leveraging our in-house precision automation and cryogenics capabilities that we were then applying in the semiconductor manufacturing market. This led us to develop and provide solutions for automated ultra-cold storage. Since then, we have expanded our life sciences offerings through internal investments and through a series of acquisitions. We now support our customers from research and clinical development to commercialization with our sample management, automated storage, vaccine cold storage and transport, as well as genomic services expertise to help our customers bring impactful and breakthrough therapies to market faster. We understand the importance of sample integrity and offer a broad portfolio of products and services supporting customers at every stage of the life cycle of samples including procurement, automated storage systems, genomic services and a multitude of sample consumables, informatics and data software, along with sample repository services. Our expertise, global footprint and leadership positions enable us to be a trusted global partner to pharmaceutical, biotechnology and life sciences research institutions. In total, we employ approximately 3,300 full-time employees, part-time employees and contingent workers worldwide as of September 30, 2024 and have sales in approximately 125 countries. We are headquartered in Burlington, Massachusetts and have operations in North America, Asia, and Europe.
Our portfolio includes product and service offerings developed by us internally, as well as obtained through acquisitions, designed to provide comprehensive capabilities to our customers, addressing their needs in sample exploration and management, automated storage, multiomics, and cold chain solutions. We continue to develop new product and service offerings and enhance existing and acquired offerings through the expertise of our research and development resources. We believe our acquisition, investment and integration approach has allowed us to accelerate internal development and significantly accelerate time to market for our life sciences solutions.
Segments
Effective October 1, 2023, we realigned our organizational structure into three reportable segments: Sample Management Solutions, Multiomics, and B Medical Systems. The segment realignment had no impact on our consolidated financial position, results of operations, or cash flows. All segment information presented is reflective of this new structure and prior period information has been recast to conform to our current period presentation. For further information on our reportable and operating segments, please refer to Note 18, Segment and Geographic Information to our Consolidated Financial Statements included under Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10‑K.
Within our Sample Management Solutions segment, we operate as a single business unit offering end-to-end sample management products and services, including Sample Repository Services, or SRS, and Core Products (Automated Stores, Cryogenic Systems, Automated Sample Tube, Consumables and Instruments, and Controlled Rate Thawing Devices). This portfolio provides customers with a high level of sample quality, security, availability, intelligence and integrity throughout the lifecycle of samples, providing customers with complete end-to-end “cold chain of custody” capabilities. We also offer expert-level consultation services to our clients throughout their experimental design and implementation processes. On July 1, 2022, we acquired Barkey Holding GmbH and its subsidiaries, or “Barkey”, a leading provider of controlled rate thawing devices for customers in the medical, biotech and pharmaceutical industries. The acquisition added innovative products and capabilities that extend our extensive cold chain of condition portfolio of products and services, while also expanding our customer reach in the fast-growing CGT space.
Within our Multiomics segment, our genomic services business advances research and development activities by providing gene sequencing, synthesis, editing and related services. We offer a comprehensive, global portfolio that we believe has both broad appeal in the life sciences industry and enables customers to select the best solution for their research and development challenges. This portfolio also offers unique solutions for key markets such as CGT, antibody development and biomarker discovery by addressing genomic complexity and throughput challenges.
Within our B Medical Systems segment, we provide temperature-controlled storage and transportation solutions that complement our cold chain capabilities, adding differentiated solutions for reliable and traceable transport of temperature-sensitive specimens worldwide. We offer end-to-end cold chain of custody capabilities for vaccines, blood components, and laboratory specimens through our portfolio of cold chain transport solutions, plasma freezers, contact shock freezers, ultra-low freezers, and real-time sample monitoring and location tracking solutions.
Business and Financial Performance
Our performance for the fiscal years ended September 30, 2024, 2023 and 2022 is as follows (in thousands):
| Year Ended September 30, |
||||||||||||
| 2024 |
2023 |
2022 |
||||||||||
| Revenue |
$ | 656,323 | $ | 665,072 | $ | 555,498 | ||||||
| Cost of revenue |
392,956 | 401,932 | 299,914 | |||||||||
| Gross profit |
263,367 | 263,140 | 255,584 | |||||||||
| Operating expenses |
||||||||||||
| Research and development |
33,525 | 33,956 | 27,542 | |||||||||
| Selling, general and administrative |
302,737 | 316,282 | 251,465 | |||||||||
| Impairment of goodwill and intangible assets |
115,975 | — | — | |||||||||
| Contingent consideration - fair value adjustments |
— | (18,549 | ) | 600 | ||||||||
| Restructuring charges |
11,808 | 4,577 | 712 | |||||||||
| Total operating expenses |
464,045 | 336,266 | 280,319 | |||||||||
| Operating loss |
(200,678 | ) | (73,126 | ) | (24,735 | ) | ||||||
| Other income (expense) |
||||||||||||
| Interest income, net |
33,177 | 43,735 | 15,697 | |||||||||
| Other income (expense), net |
178 | (1,042 | ) | (898 | ) | |||||||
| Loss before income taxes |
(167,323 | ) | (30,433 | ) | (9,936 | ) | ||||||
| Income tax (benefit) expense |
(3,153 | ) | (17,550 | ) | 1,350 | |||||||
| Loss from continuing operations |
$ | (164,170 | ) | $ | (12,883 | ) | $ | (11,286 | ) | |||
| (Loss) income from discontinued operations, net of tax |
— | (1,374 | ) | 2,144,145 | ||||||||
| Net (loss) income |
$ | (164,170 | ) | $ | (14,257 | ) | $ | 2,132,859 | ||||
Results of Operations
Fiscal Year Ended September 30, 2024 compared to Fiscal Year Ended September 30, 2023
Revenue decreased 1% for fiscal year 2024 compared to fiscal year 2023 driven by decreased revenue in the B Medical Systems segment, partially offset by increased revenue in the Sample Management Solutions and Multiomics segments. Gross margin was 40.1% for fiscal year 2024 compared to 39.6% for fiscal year 2023 driven by margin expansion in the Sample Management Solutions and Multiomics segments, partially offset by margin pressure from decreased revenue in the B Medical Systems segment. Operating expenses increased in fiscal year 2024 compared to the prior fiscal year, primarily due to the $116.0 million non-cash impairment of goodwill and intangible assets, increased restructuring and transformation costs recognized in fiscal year 2024, and a benefit of $18.5 million of fair value contingent consideration adjustments related to the B Medical Systems segment in fiscal year 2023 which did not reoccur; these increases were partially offset by decreased selling, general and administrative expenses in fiscal year 2024. We generated a net loss of $164.2 million for fiscal year 2024 compared to a net loss of $14.3 million for fiscal year 2023, primarily due to the impairment of goodwill and intangible assets, a lower income tax benefit and decreased interest income during fiscal year 2024.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of the Consolidated Financial Statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue, business combinations, intangible assets, goodwill and other long-lived assets, inventories, income taxes, and stock-based compensation. We base our estimates on historical experience and various other assumptions that we deem reasonable under the circumstances. We evaluate current and anticipated worldwide economic conditions, both in general and specific to the life sciences industry, that serve as a basis for making judgments about the carrying values of assets and liabilities that are not readily determinable based on information from other sources. Actual results may differ from these estimates and could have a material impact on our financial condition and results of operations.
We believe that the assumptions and estimates associated with the following critical accounting policies involve significant judgment and thus have the most significant potential impact on our Consolidated Financial Statements.
Revenue Recognition
We generate revenue from the sale of products and services. A description of our revenue recognition policies is included in Note 2, Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10‑K.
Although most of our sales agreements contain standard terms and conditions, certain agreements contain multiple performance obligations or non-standard terms and conditions. For customer contracts that contain more than one performance obligation, we allocate the total transaction consideration to each performance obligation based on the relative stand-alone selling price of each performance obligation within the contract. We rely on either observable standalone sales or an expected cost-plus margin approach to determine the standalone selling price of offerings, depending on the nature of the performance obligation. Performance obligations whose standalone selling price is estimated using an expected cost-plus margin approach relate to the sale of customized automated cold sample management systems and service-type warranties within the Sample Management Solutions segment.
Revenue from the sales of certain products that involve significant customization, primarily automated cold sample management systems, is recognized over time as the asset created by our performance does not have alternative use to us and there is an enforceable right to payment for performance completed to date. We recognize revenue as work progresses based on actual labor hours incurred to date as a percentage of total estimated labor hours expected to be incurred. We believe this method most appropriately depicts our efforts towards satisfaction of the performance obligation. We develop profit estimates for long-term contracts based on total revenue expected to be generated from the project and total costs anticipated to be incurred in the project. These estimates are based on a number of factors, including the degree of required product customization and the work required to be able to install the product in the customer’s existing environment, as well as our historical experience, project plans and an assessment of the risks and uncertainties inherent in the contract related to implementation delays or performance issues that may or may not be within our control. We estimate a loss on a contract by comparing total estimated contract revenue to the total estimated contract costs and recognize a loss during the period in which it becomes probable and can be reasonably estimated. We review profit estimates for long-term contracts during each reporting period and revise the estimate based on changes in circumstances.
If our judgment or estimates in revenue recognition prove incorrect, our revenue in particular periods may be adversely affected and could have a material impact on our financial condition and results of operations.
Business Combinations
We account for business acquisitions using the purchase method of accounting, in accordance with which assets acquired and liabilities assumed are recorded at their respective fair values at the acquisition date.
Significant judgment is used in determining fair values of assets acquired, liabilities assumed, and contingent consideration, as well as intangibles and their estimated useful lives. Fair value and useful life determinations require estimates of revenue growth rates, operating expenses, integration costs, obsolescence factors, discount rates and other assumptions used in computing present value. These judgments may materially impact the estimates used in allocating acquisition date fair values to assets acquired and liabilities assumed, as well as our current and future operating results. Actual results may vary from these estimates and may result in adjustments to goodwill and acquisition date fair values of assets and liabilities during a measurement period or upon a final determination of asset and liability fair value, whichever occurs first. Adjustments to fair value of assets and liabilities made after the end of the measurement period are recorded within our operating results.
Contingent consideration is recorded at fair value as measured on the date of acquisition using an appropriate valuation model, such as the Monte Carlo simulation model. The value recorded is based on estimates of future financial projections under various potential scenarios, in which the model runs many simulations based on comparable companies’ growth rates and their implied volatility. Our estimates of forecasted revenues in the earn-out period include a consideration of current industry information, market and economic trends, historical results of the acquired business and other relevant factors. These cash flow projections are discounted with a risk-adjusted rate. Each reporting period until such contingent amounts are earned, the fair value of the liability is remeasured based on changes to the underlying assumptions. The estimates used to determine the fair value of the contingent consideration liability are subject to significant judgment and given the inherent uncertainties in making these estimates, actual results are likely to differ from the amounts originally recorded and could be materially different.
Intangible Assets, Goodwill and Other Long-Lived Assets
We have identified intangible assets and recorded significant goodwill as a result of our acquisitions. Intangible assets other than goodwill are valued based on estimated future cash flows and amortized over the assets' estimated useful lives. Goodwill is tested for impairment annually or more often if impairment indicators are present, at the reporting unit level. Intangible assets other than goodwill and long-lived assets are subject to impairment testing if events and circumstances indicate that the carrying amount of an asset or a group of assets may not be recoverable.
In performing a quantitative test for impairment, annually or in the interim period if required based on qualitative factors (as described further in Note 2, Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10‑K), we determine fair values of our reporting units based on an income approach in accordance with the discounted cash flow method, or “DCF Method”. The DCF Method is based on projected future cash flows and terminal value estimates discounted to their present value. The key inputs used in the DCF Method include revenue growth rates, forecast gross profit margins, operating expenses and capital expenditures, terminal period growth rate, economic and market trends, and discount rate. We derive discount rates that are commensurate with the risks and uncertainties inherent in the respective reporting units and our internally developed projections of future cash flows.
Application of the goodwill impairment test requires judgment based on market and operational conditions at the time of the evaluation, including management’s best estimate of the reporting unit’s future business activity and the related estimates and assumptions of future cash flows from the assets that include the associated goodwill. Different assumptions for inputs used in the DCF Method could result in different estimates of reporting unit fair value as of each testing date.
In the event the financial performance of one of our business segments does not meet our expectations in the future, we experience a prolonged macro or market downturn, or there are other negative revisions to key assumptions used in our DCF Method analysis, we may be required to perform additional impairment analyses and could be required to recognize a non-cash impairment charge.
We are required to test long-lived assets, other than goodwill, for impairment when impairment indicators are present. For purposes of this test, long-lived assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities. If we determine that indicators of potential impairment are present, we assess the recoverability of the long-lived asset group by comparing its undiscounted future cash flows to its carrying value. If the carrying value of the long-lived asset group exceeds its future cash flows, we determine fair value of the individual assets within the long-lived asset group to assess potential impairment. If the aggregate fair value of the individual assets of the group are less than their carrying value, an impairment loss is recognized for an amount in excess of the group’s aggregate carrying value over its fair value. The loss is allocated to the assets within the group based on their relative carrying values, with no asset reduced below its fair value.
Inventory
We state our inventory at the lower of cost or market and make adjustments to reduce the inventory cost to its net realizable value through estimated reserves for excess or obsolete inventory. The reserves are established for the difference between the cost of inventory and its estimated market value based on assumptions related to future demand and market conditions. We fully reserve for inventories and non-cancelable purchase orders for inventory deemed obsolete. We perform periodic reviews of our inventory to identify excess inventory on hand. We compare on-hand inventory balances to anticipated inventory usage based on our recent historical activity and forecasted demand for our products developed through our planning systems and sales and marketing inputs.
We adjust the reserves for excess or obsolete inventory and record additional inventory write-downs based on unfavorable changes in estimated customer demand or actual market conditions that differ from management's previous projections.
Deferred Income Taxes
We evaluate the realizability of our deferred tax assets and assess the need for a valuation allowance on a quarterly basis. We operate in numerous countries under many legal forms and, as a result, we are subject to the jurisdiction of numerous domestic and foreign tax authorities. We evaluate the profitability of our operations in each jurisdiction on a historic cumulative basis and on a forward-looking basis, while carefully considering carry-forward periods of tax attributes and ongoing tax planning strategies in assessing the need for the valuation allowance. During fiscal year 2024, we recorded $5.6 million U.S. federal and state valuation allowances against deferred tax assets, resulting in a total U.S. valuation allowance of $7.7 million as of September 30, 2024. We also maintain valuation allowances against net deferred tax assets in foreign jurisdictions totaling $40.2 million as of September 30, 2024.
Stock-Based Compensation
We measure compensation cost for all employee stock awards at fair value on the date of grant and recognize compensation expense over the service period for awards expected to vest. The fair value of restricted stock units is determined based on the number of shares granted and the closing price of our common stock quoted on the Nasdaq Global Select Market on the date of grant. In addition, for stock-based awards where vesting is dependent upon achieving certain operating performance goals, we estimate the likelihood of achieving the performance goals. Each reporting period we review and revise, as needed, estimated achievement of performance goals as part of calculating compensation cost. Actual results may differ from our estimates.
Recently Issued Accounting Pronouncements
For a summary of recently issued accounting pronouncements applicable to our Consolidated Financial Statements which is incorporated here by reference, please refer to Note 2, Summary of Significant Accounting Policies in the Notes to the Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10‑K.
RESULTS OF OPERATIONS
Please refer to the commentary provided below for further discussion and analysis of the factors contributing to our results from operations for the twelve months ended September 30, 2024 and 2023. A comparison of our results for the fiscal year ended September 30, 2023 to the fiscal year ended September 30, 2022 is included in Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the fiscal year ended September 30, 2023 filed with the SEC on November 21, 2023.
Non-GAAP Financial Measures
Non-GAAP financial measures are used in addition to and in conjunction with results presented in accordance with GAAP and should not be relied upon to the exclusion of GAAP financial measures. Management adjusts the GAAP results for the impact of amortization of intangible assets, purchase accounting impact on inventory, transformation and rebranding costs, restructuring charges, goodwill and intangible asset impairments, fair value adjustments to contingent consideration, governance-related matters, merger and acquisition costs and costs related to share repurchase, and other unallocated corporate expenses to provide investors better perspective on the results of operations which the Company believes is more comparable to the similar analysis provided by its peers. Management also excludes special charges and gains, such as gains and losses from the sale of assets, certain tax benefits and charges, as well as other gains and charges that are not representative of the normal operations of the business. Management strongly encourages investors to review our financial statements and publicly filed reports in their entirety and not rely on any single measure. A reconciliation of each non-GAAP measure to the most nearly comparable GAAP measure is included under "Operating Income (Loss)" and "Gross Margin" below.
Revenue
Our revenue performance for the fiscal years ended September 30, 2024, 2023, and 2022 is as follows (in thousands, except percentages):
| Year Ended September 30, |
||||||||||||||||||||
| % Change |
||||||||||||||||||||
| 2024 |
2023 |
2022 |
2024 v. 2023 |
2023 v. 2022 |
||||||||||||||||
| Sample Management Solutions |
$ | 318,646 | $ | 303,654 | $ | 304,561 | 4.9 | % | (0.3 | )% | ||||||||||
| Multiomics |
254,552 | 248,296 | 250,937 | 2.5 | % | (1.1 | )% | |||||||||||||
| B Medical Systems |
83,125 | 113,122 | — | (26.5 | )% | NM | ||||||||||||||
| Total revenue |
$ | 656,323 | $ | 665,072 | $ | 555,498 | (1.3 | )% | NM | |||||||||||
Fiscal Year Ended September 30, 2024 compared to Fiscal Year Ended September 30, 2023
Revenue decreased 1% in fiscal year 2024 compared to the prior fiscal year driven by a decline in our B Medical Systems segment, partially offset by revenue growth in our Sample Management Solutions and Multiomics segments.
Our B Medical Systems segment revenue decreased 27% in fiscal year 2024 compared to the prior fiscal year, primarily due to lower order volume for cold chain equipment.
Our Sample Management Solutions segment revenue increased 5% in fiscal year 2024 compared to the prior fiscal year driven by broad based revenue growth across most product lines in both the Sample Repository Services and Core Products businesses.
Our Multiomics segment revenue increased 3% in fiscal year 2024 compared to the prior fiscal year driven by revenue growth in Next Generation Sequencing and Gene Synthesis, partially offset by a decline in Sanger sequencing services.
Revenue generated outside the United States was $290.1 million, or 44% of total revenue, for fiscal year 2024 compared to $310.0 million, or 47% of total revenue, in the prior fiscal year.
Operating Income (Loss)
Our operating performance for the fiscal years ended September 30, 2024, 2023 and 2022 is as follows (in thousands, except percentages):
| Sample Management Solutions |
Multiomics |
B Medical Systems |
||||||||||||||||||||||||||||||||||
| Year Ended September 30, | Year Ended September 30, | Year Ended September 30, | ||||||||||||||||||||||||||||||||||
| 2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
||||||||||||||||||||||||||||
| Revenue: |
$ | 318,646 | $ | 303,654 | $ | 304,561 | $ | 254,552 | $ | 248,296 | $ | 250,937 | $ | 83,125 | $ | 113,122 | $ | — | ||||||||||||||||||
| Operating income (loss): |
||||||||||||||||||||||||||||||||||||
| Operating income (loss) |
$ | 6,383 | $ | (5,633 | ) | $ | 22,335 | $ | (12,152 | ) | $ | (18,652 | ) | $ | (518 | ) | $ | (25,949 | ) | $ | (20,757 | ) | $ | — | ||||||||||||
| Amortization of completed technology |
3,909 | 2,973 | 1,631 | 4,157 | 4,874 | 5,693 | 16,704 | 10,647 | — | |||||||||||||||||||||||||||
| Purchase accounting impact on inventory |
— | — | — | — | — | — | — | 9,664 | — | |||||||||||||||||||||||||||
| Amortization of intangible assets other than completed technology |
154 | 311 | 5 | — | — | 340 | — | 1,366 | — | |||||||||||||||||||||||||||
| Transformation(1) and rebranding costs |
395 | — | — | — | — | — | 3,576 | — | — | |||||||||||||||||||||||||||
| Other adjustments |
— | — | (1 | ) | — | (1 | ) | (483 | ) | — | (1 | ) | — | |||||||||||||||||||||||
| Total adjusted operating income (loss) |
$ | 10,841 | $ | (2,349 | ) | $ | 23,970 | $ | (7,995 | ) | $ | (13,779 | ) | $ | 5,032 | $ | (5,669 | ) | $ | 919 | $ | — | ||||||||||||||
| Operating margin |
2.0 | % | (1.9 | )% | 7.3 | % | (4.8 | )% | (7.5 | )% | (0.2 | )% | (31.2 | )% | (18.3 | )% | NM | |||||||||||||||||||
| Adjusted operating margin |
3.4 | % | (0.8 | )% | 7.9 | % | (3.1 | )% | (5.5 | )% | 2.0 | % | (6.8 | )% | 0.8 | % | NM | |||||||||||||||||||
| Segment |
Corporate |
Azenta Total |
||||||||||||||||||||||||||||||||||
| Year Ended September 30, | Year Ended September 30, | Year Ended September 30, | ||||||||||||||||||||||||||||||||||
| 2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
||||||||||||||||||||||||||||
| Revenue: |
$ | 656,323 | $ | 665,072 | $ | 555,498 | $ | — | $ | — | $ | — | $ | 656,323 | $ | 665,072 | $ | 555,498 | ||||||||||||||||||
| Operating income (loss): |
||||||||||||||||||||||||||||||||||||
| Operating income (loss) |
$ | (31,718 | ) | $ | (45,042 | ) | $ | 21,817 | $ | (168,960 | ) | $ | (28,084 | ) | $ | (46,552 | ) | $ | (200,678 | ) | $ | (73,126 | ) | $ | (24,735 | ) | ||||||||||
| Amortization of completed technology |
24,770 | 18,494 | 7,324 | — | — | — | 24,770 | 18,494 | 7,324 | |||||||||||||||||||||||||||
| Purchase accounting impact on inventory |
— | 9,664 | — | — | — | — | — | 9,664 | — | |||||||||||||||||||||||||||
| Amortization of intangible assets other than completed technology |
154 | 1,677 | 345 | 26,346 | 28,207 | 24,620 | 26,500 | 29,884 | 24,965 | |||||||||||||||||||||||||||
| Transformation(1) and rebranding costs |
3,971 | — | — | 9,885 | (49 | ) | 2,741 | 13,856 | (49 | ) | 2,741 | |||||||||||||||||||||||||
| Restructuring charges |
— | — | — | 11,808 | 4,577 | 712 | 11,808 | 4,577 | 712 | |||||||||||||||||||||||||||
| Impairment of goodwill and intangible assets |
115,975 | — | — | 115,975 | — | — | ||||||||||||||||||||||||||||||
| Contingent consideration - fair value adjustments |
— | — | — | — | (18,549 | ) | 600 | — | (18,549 | ) | 600 | |||||||||||||||||||||||||
| Merger and acquisition costs and costs related to share repurchase(2) |
— | — | — | 4,874 | 13,842 | 17,329 | 4,874 | 13,842 | 17,329 | |||||||||||||||||||||||||||
| Other adjustments |
— | (2 | ) | (484 | ) | — | 1 | — | — | (1 | ) | (484 | ) | |||||||||||||||||||||||
| Total adjusted operating income (loss) |
$ | (2,823 | ) | $ | (15,209 | ) | $ | 29,002 | $ | (72 | ) | $ | (55 | ) | $ | (550 | ) | $ | (2,895 | ) | $ | (15,264 | ) | $ | 28,452 | |||||||||||
| Operating margin |
(4.8 | )% | (6.8 | )% | 3.9 | % | (30.6 | )% | (11.0 | )% | (4.5 | )% | ||||||||||||||||||||||||
| Adjusted operating margin |
(0.4 | )% | (2.3 | )% | 5.2 | % | (0.4 | )% | (2.3 | )% | 5.1 | % | ||||||||||||||||||||||||
| (1) | Transformation costs represent non-recurring expenses for strategic projects with anticipated long-term benefits to the Company focused on cost reduction and productivity improvement that do not meet the definition of restructuring charges. These costs are directed at simplifying, standardizing, streamlining, and optimizing the Company’s operations, processes and systems to permanently alter the Company’s operations for the long term. For a project to be considered transformational, successful completion of the project must be expected to bring long-term material benefits to the organization and involve significant changes to process and/or underlying technology. Transformation costs in the period result from actions taken as part of the Company’s 2024 cost reduction plan, and primarily relate to one time asset write-downs associated with changes in technology, one time inventory write-downs relating to restructuring actions taken in the period, and third-party consulting costs associated with process and systems re-design. |
| (2) |
Includes expenses related to governance-related matters. |
Operating income for the Sample Management Solutions segment was $6.4 million for fiscal year 2024 compared to an operating loss of $5.6 million in the prior fiscal year. The Sample Management Solutions segment operating margin was 2.0%, an increase of 386 basis points year over year. The increases in operating income and operating margin were primarily driven by higher revenue, supported by operating leverage and cost reduction initiatives. Adjusted operating income for the Sample Management Solutions segment was $10.8 million for fiscal year 2024 compared to adjusted operating loss of $2.3 million in the prior fiscal year. Adjusted operating margin for the Sample Management Solutions segment was 3.4%, an increase of 418 basis points year over year. Adjusted operating income (loss) and margin exclude the impact of amortization of intangible assets of $4.1 million and $3.3 million for fiscal years 2024 and 2023, respectively, and transformation costs of $0.4 million for fiscal year 2024.
Operating loss for the Multiomics segment was $12.2 million for fiscal year 2024 compared to an operating loss of $18.7 million in the prior fiscal year. The Multiomics segment operating margin was (4.8)%, an increase of 274 basis points year over year. The decrease in operating loss and increase in operating margin were primarily driven by higher revenue and gross profit, supported by operating leverage. Adjusted operating loss for the Multiomics segment was $8.0 million for fiscal year 2024 compared to adjusted operating loss of $13.8 million in the prior fiscal year. Adjusted operating margin for the Multiomics segment was (3.1)%, an increase of 241 basis points year over year. Adjusted operating loss and margin exclude the impact of amortization related to completed technology of $4.2 million and $4.9 million for fiscal years 2024 and 2023, respectively.
Operating loss for the B Medical Systems segment was $25.9 million for fiscal year 2024 compared to an operating loss of $20.8 million in the prior fiscal year. The B Medical Systems segment operating margin was (31.2)%, a decrease of 1,287 basis points year over year. The increase in operating loss and decrease in operating margin were primarily driven by lower revenue and gross profit due to lower volume of cold chain sales in the product mix, partially offset by lower operating expenses due to decreased commissions on cold chain sales and cost reduction initiatives. Adjusted operating loss for the B Medical Systems segment was $5.7 million for fiscal year 2024 compared to adjusted operating income of $0.9 million in the prior fiscal year. Adjusted operating margin for the B Medical Systems segment was (6.8)%, a decrease of 763 basis points year over year. Adjusted operating income (loss) and margin exclude the impact of amortization of intangible assets of $16.7 million and $12.0 million for fiscal years 2024 and 2023, respectively, transformation costs of $3.6 million for fiscal year 2024 and purchase accounting impact on inventory of $9.7 million for fiscal year 2023.
Gross Margin
Our gross margin performance for the fiscal years ended September 30, 2024, 2023 and 2022 is as follows (in thousands, except percentages):
| Sample Management Solutions |
Multiomics |
B Medical Systems |
Azenta Total |
|||||||||||||||||||||||||||||||||||||||||||||
| Year Ended September 30, |
Year Ended September 30, |
Year Ended September 30, |
Year Ended September 30, |
|||||||||||||||||||||||||||||||||||||||||||||
| 2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
2024 |
2023 |
2022 |
|||||||||||||||||||||||||||||||||||||
| Revenue |
$ | 318,646 | $ | 303,654 | $ | 304,561 | $ | 254,552 | $ | 248,296 | $ | 250,937 | $ | 83,125 | $ | 113,122 | $ | — | $ | 656,323 | $ | 665,072 | $ | 555,498 | ||||||||||||||||||||||||
| Gross profit |
142,035 | 132,806 | 140,940 | 115,434 | 109,820 | 114,644 | 5,895 | 20,514 | — | 263,367 | 263,140 | 255,584 | ||||||||||||||||||||||||||||||||||||
| Adjustments: |
— | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Amortization of completed technology |
3,909 | 2,973 | 1,631 | 4,157 | 4,874 | 5,693 | 16,704 | 10,647 | — | 24,770 | 18,494 | 7,324 | ||||||||||||||||||||||||||||||||||||
| Purchase accounting impact on inventory |
— | — | — | — | — | — | — | 9,664 | — | — | 9,664 | — | ||||||||||||||||||||||||||||||||||||
| Transformation costs(1) |
377 | — | — | — | — | — | 3,576 | — | — | 3,953 | — | — | ||||||||||||||||||||||||||||||||||||
| Tariff adjustment |
— | — | — | — | — | (484 | ) | — | — | — | — | — | (484 | ) | ||||||||||||||||||||||||||||||||||
| Other adjustments |
— | — | 7 | — | — | 295 | — | (1 | ) | — | — | (1 | ) | 302 | ||||||||||||||||||||||||||||||||||
| Adjusted gross profit |
$ | 146,321 | $ | 135,779 | $ | 142,578 | $ | 119,591 | $ | 114,694 | $ | 120,148 | $ | 26,175 | $ | 40,824 | $ | — | $ | 292,090 | $ | 291,297 | $ | 262,726 | ||||||||||||||||||||||||
| Gross margin |
44.6 | % | 43.7 | % | 46.3 | % | 45.3 | % | 44.2 | % | 45.7 | % | 7.1 | % | 18.1 | % | NM | 40.1 | % | 39.6 | % | 46.0 | % | |||||||||||||||||||||||||
| Adjusted gross margin |
45.9 | % | 44.7 | % | 46.8 | % | 47.0 | % | 46.2 | % | 47.9 | % | 31.5 | % | 36.1 | % | NM | 44.5 | % | 43.8 | % | 47.3 | % | |||||||||||||||||||||||||
| (1) | Transformation costs represent non-recurring expenses for strategic projects with anticipated long-term benefits to the Company focused on cost reduction and productivity improvement that do not meet the definition of restructuring charges. These costs are directed at simplifying, standardizing, streamlining, and optimizing the Company’s operations, processes and systems to permanently alter the Company’s operations for the long term. For a project to be considered transformational, successful completion of the project must be expected to bring long-term material benefits to the organization and involve significant changes to process and/or underlying technology. Transformation costs in the period result from actions taken as part of the Company’s 2024 cost reduction plan, and primarily relate to one time asset write-downs associated with changes in technology, one time inventory write-downs relating to restructuring actions taken in the period, and third-party consulting costs associated with process and systems re-design. |
The Sample Management Solutions segment gross margin was 44.6% for fiscal year 2024, an increase of 84 basis points compared to the prior fiscal year. Adjusted gross margin for the Sample Management Solutions segment was 45.9% for fiscal year 2024, an increase of 120 basis points compared to the prior fiscal year, driven by higher gross margin for both the Core Products and Sample Repository Services businesses. Adjusted gross margin excludes the impact of amortization related to completed technology of $3.9 million and $3.0 million for fiscal years 2024 and 2023, respectively, and transformation costs of $0.4 million for fiscal year 2024.
The Multiomics segment gross margin was 45.3% for fiscal year 2024, an increase of 112 basis points compared to the prior fiscal year. Adjusted gross margin for the Multiomics segment was 47.0% for fiscal year 2024, an increase of 79 basis points compared to the prior fiscal year, driven by higher gross margin for the Next Generation Sequencing and Gene Synthesis, partially offset by lower gross margin for Sanger sequencing services. Adjusted gross margin excludes the impact of amortization related to completed technology of $4.2 million and $4.9 million for fiscal years 2024 and 2023, respectively.
The B Medical Systems segment gross margin was 7.1% for fiscal year 2024, a decrease of 1,104 basis points compared to the prior fiscal year. Adjusted gross margin for the B Medical Systems segment was 31.5% for fiscal year 2024, a decrease of 460 basis points compared to the prior fiscal year, primarily due to lower volume of cold chain sales in the product mix. Adjusted gross margin excludes the impact of amortization related to completed technology of $16.7 million and $10.6 million for fiscal years 2024 and 2023, respectively, purchase accounting impact on inventory of $9.7 million for fiscal year 2023, and transformation costs of $3.6 million for fiscal year 2024.
Research and Development Expenses
Our research and development expense for the fiscal years ended September 30, 2024, 2023, and 2022 is as follows (in thousands, except percentages):
| Year Ended September 30, |
||||||||||||||||||||||||
| 2024 |
2023 |
2022 |
||||||||||||||||||||||
| % of Revenue |
% of Revenue |
% of Revenue |
||||||||||||||||||||||
| Sample Management Solutions |
$ | 17,122 | 5.4 | % | $ | 17,934 | 5.9 | % | $ | 15,565 | 5.1 | % | ||||||||||||
| Multiomics |
11,766 | 4.6 | % | 11,976 | 4.8 | % | 11,977 | 4.8 | % | |||||||||||||||
| B Medical Systems |
4,637 | 5.6 | % | 4,046 | 3.6 | % | — | — | ||||||||||||||||
| Total research and development expense |
$ | 33,525 | 5.1 | % | $ | 33,956 | 5.1 | % | $ | 27,542 | 5.0 | % | ||||||||||||
Total research and development expenses remained flat in fiscal year 2024 compared to fiscal year 2023, driven by cost reduction initiatives across all three business segments, primarily from decreased expenditures for external services, offset by increased research and development expenses in our B Medical Systems segment related to vaccine cold chain.
Selling, General and Administrative Expenses
Our selling, general and administrative expense for the fiscal years ended September 30, 2024, 2023, and 2022 is as follows (in thousands, except percentages):
| Year Ended September 30, |
||||||||||||||||||||||||
| 2024 |
2023 |
2022 |
||||||||||||||||||||||
| % of Revenue |
% of Revenue |
% of Revenue |
||||||||||||||||||||||
| Sample Management Solutions |
$ | 118,531 | 37.2 | % | $ | 120,495 | 39.7 | % | $ | 103,035 | 33.8 | % | ||||||||||||
| Multiomics |
115,820 | 45.5 | % | 116,491 | 46.9 | % | 103,200 | 41.1 | % | |||||||||||||||
| B Medical Systems |
27,206 | 32.7 | % | 37,225 | 32.9 | % | — | — | ||||||||||||||||
| Corporate |
41,180 | 6.3 | % | 42,071 | 6.3 | % | 45,230 | 8.1 | % | |||||||||||||||
| Total selling, general and administrative expense |
$ | 302,737 | 46.1 | % | $ | 316,282 | 47.6 | % | $ | 251,465 | 45.3 | % | ||||||||||||
Total selling, general and administrative expenses decreased $13.5 million for fiscal year 2024 compared to fiscal year 2023, driven by cost reduction initiatives across the business and lower commissions on cold chain sales in our B Medical Systems segment.
Restructuring Charges
Restructuring charges were $11.8 million for fiscal year 2024, an increase of $7.2 million from fiscal year 2023, driven by initiatives launched in fiscal year 2024. See Note 9, Restructuring, in the Notes to the Consolidated Financial Statements included in Part II, Item 8, "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
Other Income (Expense)
Interest income, net – We recorded interest income of $33.2 million for fiscal year 2024, a decrease of $10.6 million year over year, driven by decreased investments in marketable securities. See Note 5, Marketable Securities, in the Notes to the Consolidated Financial Statements included in Part II, Item 8, "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K.
Other income (expense), net – We recorded other income of $0.2 million and other expense of $1.0 million in fiscal years 2024 and 2023, respectively, primarily due to foreign exchange gains and losses.
Income Tax (Benefit) Expense
We recorded an income tax benefit on continuing operations of $3.2 million in fiscal year 2024 compared to an income tax benefit of $17.6 million in fiscal year 2023. The decreased tax benefit for the year was primarily driven by the decreased global taxable loss from operations and by $5.6 million of charges related to a valuation allowance recorded against U.S. deferred tax assets. The pretax global loss and global taxable loss are significantly different primarily due to the goodwill impairment charge which is not deductible for tax purposes. During fiscal year 2024, we repatriated approximately $455.0 million in cash from our German subsidiary. We recorded a net tax benefit in the amount of $3.2 million related to the repatriation. The benefit included $5.2 million related to deductible U.S. foreign exchange losses on the repatriation measured at the foreign exchange rate on the date of repatriation. This benefit was offset by $2.0 million of state income taxes, net of federal benefit. The tax provision impacts in fiscal year 2024 were offset by the reversal of the related deferred tax asset recorded in fiscal year 2023. Additionally, during fiscal year 2024, we reversed $2.9 million of the deferred tax asset previously established due to changes in foreign exchange rates up to the repatriation date. The impact was recorded against other comprehensive income.
Discontinued Operations
Discontinued operations in fiscal year 2022 consisted of the semiconductor automation business. On February 1, 2022, the Company completed the sale of the semiconductor automation business for $2.9 billion in cash.
There was no revenue from discontinued operations for fiscal year 2024 or fiscal year 2023. Revenue from discontinued operations was $264.4 million for fiscal year 2022. There was no net income or loss from discontinued operations for fiscal year 2024. Net loss from discontinued operations was $1.4 million for fiscal year ended 2023 and net income from discontinued operations was $2.1 billion for fiscal year 2022. The net loss from discontinued operations in fiscal year 2023 was primarily driven by adjustments to liabilities related to discontinued operations of the semiconductor cryogenics business, specifically the accrued liability for the litigation with Edwards Vacuum LLC which was recorded during the second quarter of fiscal year 2023 and is discussed in Note 19, Commitments and Contingencies in the Notes to the Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10 K. Net income from discontinued operations in fiscal year 2022 is primarily the gain on the sale of the semiconductor business. Income from discontinued operations only includes direct operating expenses incurred that (1) are clearly identifiable as costs being disposed of upon completion of the sale and (2) will not be continued by our company on an ongoing basis. Indirect expenses which supported the semiconductor automation business and semiconductor cryogenics business, and which remained as part of the continuing operations, are not reflected in income from discontinued operations. See Note 3, Discontinued Operations in the Notes to the Consolidated Financial Statements included in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10 K.
LIQUIDITY AND CAPITAL RESOURCES
The Consolidated Statement of Cash Flows for the year ended September 30, 2023 has been revised to correct for prior period errors as discussed in Note 2, Summary of Significant Accounting Policies – Revisions to Previously Issued Financial Statements and Financial Information to our Consolidated Financial Statements included under Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10 K. Accordingly, this MD&A reflects the effects of the revisions.
As of September 30, 2024, we had cash, cash equivalents, and restricted cash of $321.0 million, marketable securities of $200.6 million, and stockholders’ equity of $1.8 billion. Net cash provided by operating activities was $50.3 million and $7.2 million for fiscal years 2024 and 2023, respectively. We incurred a net loss of $164.2 million and $14.3 million for fiscal years 2024 and 2023, respectively. The net loss for fiscal year 2024 is primarily due to $116.0 million non-cash impairment of goodwill and intangible assets. We believe that our current cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements for at least one year from the date of this Annual Report on Form 10-K and for the foreseeable future thereafter. The current global economic environment makes it difficult for us to predict longer-term liquidity requirements with sufficient certainty. We may be unable to obtain any additional financing that may be required on terms favorable to us, if at all. If adequate funds are not available to us on acceptable terms or otherwise, we may be unable to successfully develop or enhance products and services, respond to competitive pressures, or take advantage of acquisition opportunities, any of which could have a material adverse effect on our business, financial condition and operating results.
Cash Flows and Liquidity
The discussion of our cash flows and liquidity that follows is stated on a total company consolidated basis and excludes the impact of discontinued operations.
Our cash and cash equivalents, restricted cash and marketable securities as of September 30, 2024 and 2023 consist of the following (in thousands):
| September 30, 2024 |
September 30, 2023 |
|||||||
| Cash and cash equivalents |
$ | 310,929 | $ | 678,910 | ||||
| Restricted cash |
10,061 | 5,135 | ||||||
| Short-term marketable securities |
151,162 | 338,873 | ||||||
| Long-term marketable securities |
49,454 | 111,338 | ||||||
| $ | 521,606 | $ | 1,134,256 | |||||
As of September 30, 2024, we had $146 million of cash, cash equivalents and restricted cash held outside of the United States. If these funds are needed for U.S. operations, we would need to repatriate these funds. Based on current U.S. tax laws, any repatriation in the future would likely not result in U.S. federal income tax. Our marketable securities are generally readily convertible to cash without a material adverse impact.
Fiscal Year Ended September 30, 2024 compared to Fiscal Year Ended September 30, 2023
Our cash flows for fiscal years 2024 and 2023 were as follows (in thousands):
| Year Ended September 30, |
||||||||
| 2024 |
2023 |
|||||||
| Net cash provided by operating activities |
$ | 50,289 | $ | 7,158 | ||||
| Net cash provided by investing activities |
224,739 | 431,384 | ||||||
| Net cash used in financing activities |
(659,207 | ) | (840,459 | ) | ||||
| Effects of exchange rate changes on cash and cash equivalents |
21,124 | 44,666 | ||||||
| Net decrease in cash, cash equivalents and restricted cash |
$ | (363,055 | ) | $ | (357,251 | ) | ||
Operating Activities
Cash flows from operating activities can fluctuate significantly from period to period as earnings, working capital needs and the timing of payments for income taxes, restructuring activities and other charges impact reported cash flows.
Cash inflows from operating activities for fiscal year 2024 were $50.3 million, primarily due to improved inventory management and decreased selling, general and administrative expenses as a result of our cost savings plans and transformation initiatives.
Cash inflows from operating activities for fiscal year 2023 were $7.2 million, primarily resulted from collections on accounts receivable, and efficient working capital management partially offset by payment of retention bonuses and cash settled stock-based awards, as well as state income taxes resulting from the sale of the semiconductor automation business.
Investing Activities
Cash flows from investing activities consist primarily of proceeds from divestitures, cash used for acquisitions, capital expenditures and purchase of marketable securities as well as cash proceeds generated from sales and maturities of marketable securities.
Cash inflows from investing activities were $224.7 million for fiscal year 2024 and consisted of $666.2 million of sales and maturities of marketable securities, partially offset by $405.6 million for purchases of marketable securities and $38.4 million of capital expenditures.
Cash inflows from investing activities were $431.4 million for fiscal year 2023 and consisted of $1.1 billion of sales and maturities of marketable securities, partially offset by $236.2 million for purchases of marketable securities and $386.5 million paid for the acquisition of B Medical and Ziath.
Financing Activities
Cash outflows for financing activities were $659.2 million and $840.5 million for fiscal years 2024 and 2023, respectively, which primarily consisted of cash outflows for share repurchases.
China Facility
In April 2019, we committed to construct a facility in Suzhou China, to consolidate the Suzhou operations of our genomic services business and provide infrastructure to support future growth. The facility is being constructed in two phases. Construction of phase one of the facility was completed in fiscal year 2023 with a total cost of $43.0 million. As of September 30, 2024, we have incurred $3.2 million in costs for the construction of phase two which we expect to complete in the first quarter of fiscal year 2026 with a total cost of $15.4 million.
Capital Resources
As of September 30, 2024 and September 30, 2023, we have no outstanding debt on our balance sheet.
Dividends
Dividends are declared at the discretion of our Board of Directors and depend on actual cash flow from operations, our financial condition, debt service and capital requirements and any other factors our Board of Directors may consider relevant.
Since the completion of the sale of the semiconductor automation business on February 1, 2022 we have not paid a quarterly dividend and do not have plans to pay any dividends at this time. During fiscal year 2022, prior to the sale, we paid a $0.10 per share quarterly dividend totaling $7.5 million in December 2021.
Share Repurchase Program
On November 4, 2022, our Board of Directors approved an authorization to repurchase up to $1.5 billion of our common stock (the “2022 Repurchase Authorization”). As part of the 2022 Repurchase Authorization, we entered into an accelerated share repurchase (“ASR”) agreement for the repurchase of $500.0 million of our common stock on November 23, 2022. We received delivery of, and retired, 10.1 million shares of common stock under the ASR agreement, which terminated on April 3, 2023. In April 2023, other arrangements commenced under the 2022 Repurchase Authorization with the intent of repurchasing the remaining $1.0 billion of shares of our common stock through open market repurchases. As of September 30, 2024 we have repurchased and retired 19.9 million shares of common stock for $1.0 billion in open market repurchases. Through the ASR agreement and open market repurchases, as of September 30, 2024, we have repurchased and retired 30.0 million shares of common stock for the full $1.5 billion approved under the 2022 Repurchase Authorization and no authorization is available for additional repurchases.
See Note 12, Stockholders’ Equity in the Notes to the Consolidated Financial Statements included in Part II, Item 8 of this Annual Report on Form 10-K for additional information about the share repurchase authorization.
Contractual Obligations and Requirements
At September 30, 2024, we had non-cancelable commitments of $69.9 million, including purchase orders for inventory of $53.1 million, and information technology related commitments of $16.8 million.
Off-Balance Sheet Arrangements
As of September 30, 2024, we had no obligation, assets or liabilities which would be considered off-balance sheet arrangements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are exposed to a variety of market risks, including changes in interest rates affecting the return on our cash and cash equivalents, restricted cash and short-term and long-term investments and fluctuations in foreign currency exchange rates.
Interest Rate Exposure
Our cash and cash equivalents and restricted cash consist principally of money market securities which are short-term in nature. At September 30, 2024 and 2023, our aggregate short-term and long-term investments were $200.6 million and $450.2 million, respectively, and consisted mostly of highly rated corporate debt securities, and U.S. government backed securities. At September 30, 2024 and 2023 the net unrealized loss position on marketable securities was $0.3 million and $6.9 million, respectively, which is included in “Accumulated other comprehensive income (loss)” in the Consolidated Balance Sheets. A hypothetical 100 basis point change in interest rates would result in a $6.6 million annual change in interest income earned in fiscal year 2024.
As of September 30, 2024 and September 30, 2023, we had no outstanding debt on our balance sheet.
Currency Rate Exposure
We have transactions and balances denominated in currencies other than the functional currency of the transacting entity. Most of these transactions carrying foreign exchange risk are in Germany, the United Kingdom, Luxembourg, and China. Sales in currencies other than the U.S. dollar were 26% and 24%, respectively, of our total sales for fiscal years ended September 30, 2024 and 2023. These sales were made primarily by our foreign subsidiaries, which have cost structures that substantially align with the currency of sale.
In the normal course of our business, we have liquid assets denominated in non-functional currencies which include cash, short-term advances between our legal entities and accounts receivable which are subject to foreign currency exposure. Such balances were approximately $63.9 million and $157.8 million, respectively, at September 30, 2024 and 2023, and primarily relate to the Euro, British Pound, and the Chinese yuan. We mitigate the impact of potential currency translation losses on these short-term intercompany advances by the timely settlement of each transaction, generally within 30 days. We also utilize forward contracts to mitigate our exposures to currency movement. We incurred foreign currency losses of $3.2 million and $4.2 million, respectively, in fiscal years 2024 and 2023, which related to the currency fluctuation on these balances between the time the transaction occurred and the ultimate settlement of the transaction. A hypothetical 10% change in foreign exchange rates would result in an approximate change of $0.4 million in our net income during the fiscal year ending September 30, 2024.
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Azenta, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Azenta, Inc. and its subsidiaries (the "Company") as of September 30, 2024 and 2023, and the related consolidated statements of operations, of comprehensive income (loss), of changes in stockholders’ equity and of cash flows for each of the three years in the period ended September 30, 2024, including the related notes (collectively referred to as the "consolidated financial statements"). We also have audited the Company's internal control over financial reporting as of September 30, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of September 30, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended September 30, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of September 30, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO because a material weakness in internal control over financial reporting existed as of that date as the Company did not design and maintain effective controls related to the review of the statement of cash flows.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness referred to above is described in Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A. We considered this material weakness in determining the nature, timing, and extent of audit tests applied in our audit of the 2024 consolidated financial statements, and our opinion regarding the effectiveness of the Company’s internal control over financial reporting does not affect our opinion on those consolidated financial statements.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in management's report referred to above. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Goodwill Impairment Interim Assessments - B Medical Systems Reporting Unit
As described in Notes 2 and 8 to the consolidated financial statements, the Company’s consolidated goodwill balance was $691.4 million as of September 30, 2024, and the goodwill associated with the B Medical Systems reporting unit was zero. Goodwill is tested for impairment annually or more often if impairment indicators are present at the reporting unit level. An impairment loss is recognized for the amount by which the reporting unit’s carrying value exceeds its fair value, up to the total amount of goodwill allocated to the reporting unit. Changes to the Company’s operating segments effective October 1, 2023 resulted in a change to the Company’s reporting units, which are aligned to the Company’s operating and reportable segments. Management tested its reporting units for potential impairment immediately before and after the segment realignment and concluded that the estimated fair value of each reporting unit exceeded its respective carrying value. The B Medical Systems reportable segment was allocated $109 million of goodwill as of October 1, 2023. During the second quarter of fiscal year 2024, the Company concluded it was more likely than not the fair value of the Company’s B Medical Systems segment was less than its carrying amount and completed a quantitative goodwill impairment test. The results of the Company’s quantitative goodwill impairment analyses as of March 31, 2024 indicated an impairment of all of the goodwill allocated within its B Medical Systems reporting unit. The estimated fair value of the reporting unit was derived using the income approach, specifically the discounted cash flow method and reflect management’s assumptions regarding revenue growth rates, forecast gross profit margins, operating expenses, capital expenditures, discount rates, terminal period growth rates, economic and market trends, and other expectations about the anticipated operating results of its reporting units.
The principal considerations for our determination that performing procedures relating to the interim goodwill impairment assessments of the B Medical Systems reporting unit is a critical audit matter are (i) the significant judgment by management when developing the fair value estimates of the B Medical Systems reporting unit; (ii) a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating management’s significant assumptions related to revenue growth rates, forecast gross profit margins, operating expenses, discount rates, and terminal period growth rates; and (iii) the audit effort involved the use of professionals with specialized skill and knowledge.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to management’s goodwill impairment assessments, including controls over the valuation of the B Medical Systems reporting unit. These procedures also included, among others (i) testing management’s process for developing the fair value estimates of the B Medical Systems reporting unit; (ii) evaluating the appropriateness of the discounted cash flow method used by management; (iii) testing the completeness and accuracy of the underlying data used in the discounted cash flow method; and (iv) evaluating the reasonableness of the significant assumptions used by management related to revenue growth rates, forecast gross profit margins, operating expenses, discount rates, and terminal period growth rates. Evaluating management’s assumptions related to revenue growth rates, forecast gross profit margins, operating expenses, and terminal period growth rates involved evaluating whether the assumptions used by management were reasonable considering (i) the current and past performance of the B Medical Systems reporting unit; (ii) the consistency with external market and industry data; and (iii) whether the assumptions were consistent with evidence obtained in other areas of the audit. Professionals with specialized skill and knowledge were used to assist in evaluating (i) the appropriateness of the discounted cash flow method and (ii) the reasonableness of the assumptions related to the discount rates and terminal period growth rates.
/s/
November 26, 2024
We have served as the Company’s auditor since 2016.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
| September 30, | September 30, | |||||||
| 2024 | 2023 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Short-term marketable securities | ||||||||
| Accounts receivable, net of allowance for expected credit losses ($ and $, respectively) | ||||||||
| Inventories | ||||||||
| Derivative asset | ||||||||
| Short-term restricted cash | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Property, plant and equipment, net | ||||||||
| Long-term marketable securities | ||||||||
| Long-term deferred tax assets | ||||||||
| Operating lease right-of-use assets | ||||||||
| Goodwill | ||||||||
| Intangible assets, net | ||||||||
| Other assets | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders' equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Deferred revenue | ||||||||
| Accrued warranty and retrofit costs | ||||||||
| Accrued compensation and benefits | ||||||||
| Accrued customer deposits | ||||||||
| Accrued VAT payable | ||||||||
| Accrued income taxes payable | ||||||||
| Accrued expenses and other current liabilities | ||||||||
| Total current liabilities | ||||||||
| Long-term tax reserves | ||||||||
| Long-term deferred tax liabilities | ||||||||
| Long-term operating lease liabilities | ||||||||
| Other long-term liabilities | ||||||||
| Total liabilities | ||||||||
| Stockholders' equity | ||||||||
| Preferred stock, $ par value - shares authorized, shares issued or outstanding | ||||||||
| Common stock, $ par value - shares authorized, shares issued and shares outstanding at September 30, 2024, shares issued and shares outstanding at September 30, 2023 | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| Treasury stock, at cost - shares at September 30, 2024 and September 30, 2023 | ( | ) | ( | ) | ||||
| Retained earnings | ||||||||
| Total stockholders' equity | ||||||||
| Total liabilities and stockholders' equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| Year Ended September 30, |
||||||||||||
| 2024 |
2023 |
2022 |
||||||||||
| Revenue |
||||||||||||
| Products |
$ | $ | $ | |||||||||
| Services |
||||||||||||
| Total revenue |
||||||||||||
| Cost of revenue |
||||||||||||
| Products |
||||||||||||
| Services |
||||||||||||
| Total cost of revenue |
||||||||||||
| Gross profit |
||||||||||||
| Operating expenses |
||||||||||||
| Research and development |
||||||||||||
| Selling, general and administrative |
||||||||||||
| Impairment of goodwill and intangible assets |
||||||||||||
| Contingent consideration - fair value adjustments |
( |
) | ||||||||||
| Restructuring charges |
||||||||||||
| Total operating expenses |
||||||||||||
| Operating loss |
( |
) | ( |
) | ( |
) | ||||||
| Other income (expense) |
||||||||||||
| Interest income, net |
||||||||||||
| Other income (expense), net |
( |
) | ( |
) | ||||||||
| Loss before income taxes |
( |
) | ( |
) | ( |
) | ||||||
| Income tax (benefit) expense |
( |
) | ( |
) | ||||||||
| Loss from continuing operations |
( |
) | ( |
) | ( |
) | ||||||
| Income (loss) from discontinued operations, net of tax |
( |
) | ||||||||||
| Net (loss) income |
$ | ( |
) | $ | ( |
) | $ | |||||
| Basic net (loss) income per share: |
||||||||||||
| Loss from continuing operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| (Loss) income from discontinued operations, net of tax |
( |
) | ||||||||||
| Net (loss) income per share |
$ | ( |
) | $ | ( |
) | $ | |||||
| Diluted net (loss) income per share: |
||||||||||||
| Loss from continuing operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| (Loss) income from discontinued operations, net of tax |
( |
) | ||||||||||
| Diluted net (loss) income per share |
$ | ( |
) | $ | ( |
) | $ | |||||
| Weighted average shares used in computing net income (loss) per share: |
||||||||||||
| Basic |
||||||||||||
| Diluted |
||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(In thousands)
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Net (loss) income | $ | ( | ) | $ | ( | ) | $ | |||||
| Other comprehensive income (loss), net of tax: | ||||||||||||
| Foreign currency translation reclassification adjustments included in income from discontinued operations (Note 2) | ( | ) | ||||||||||
| Net investment hedge currency translation adjustment, net of tax effects of $, $(), and $ for the fiscal years 2024, 2023, and 2022 respectively | ( | ) | ( | ) | ||||||||
| Foreign currency translation adjustments | ( | ) | ||||||||||
| Changes in unrealized losses on marketable securities, net of tax effects of $(), $, and $() for the fiscal years 2024, 2023, and 2022 respectively | ( | ) | ||||||||||
| Actuarial gain (loss) on pension plans, net of tax effects of $, $(), and $() for the fiscal years 2024, 2023, and 2022, respectively | ( | ) | ||||||||||
| Total other comprehensive income (loss), net of tax | ( | ) | ||||||||||
| Comprehensive (loss) income | $ | ( | ) | $ | $ | |||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| Year Ended September 30, |
||||||||||||
| 2024 |
2023 |
2022 |
||||||||||
| Cash flows from operating activities |
||||||||||||
| Net (loss) income |
$ | ( |
) | $ | ( |
) | $ | |||||
| Adjustments to reconcile net (loss) income to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation and amortization |
||||||||||||
| Impairment of goodwill and intangible assets |
||||||||||||
| Property, plant and equipment and other asset write-offs |
— | — | ||||||||||
| Inventory write-downs |
— | — | ||||||||||
| Other non-cash charges related to restructuring and transformation |
||||||||||||
| Stock-based compensation |
||||||||||||
| Contingent consideration adjustment |
( |
) | ||||||||||
| Amortization and accretion on marketable securities |
( |
) | ( |
) | ( |
) | ||||||
| Deferred income taxes |
( |
) | ( |
) | ||||||||
| Loss on extinguishment of debt |
||||||||||||
| Purchase accounting impact on inventory |
||||||||||||
| Loss (gain) on disposals of property, plant and equipment |
( |
) | ||||||||||
| Gain on divestiture, net of tax |
( |
) | ||||||||||
| Fees paid stemming from divestiture |
( |
) | ||||||||||
| Taxes paid stemming from divestiture |
( |
) | ||||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
( |
) | ( |
) | ||||||||
| Inventories |
( |
) | ||||||||||
| Accounts payable |
( |
) | ( |
) | ||||||||
| Deferred revenue |
( |
) | ( |
) | ||||||||
| Accrued warranty and retrofit costs |
( |
) | ||||||||||
| Accrued compensation and tax withholdings |
( |
) | ( |
) | ||||||||
| Other assets and liabilities |
( |
) | ( |
) | ||||||||
| Net cash provided by (used in) operating activities |
( |
) | ||||||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property, plant and equipment |
( |
) | ( |
) | ( |
) | ||||||
| Purchases of technology intangibles |
( |
) | ||||||||||
| Purchases of marketable securities and other investments |
( |
) | ( |
) | ( |
) | ||||||
| Sales and maturities of marketable securities |
||||||||||||
| Proceeds from divestiture, net of cash transferred |
||||||||||||
| Net investment hedge settlement |
||||||||||||
| Acquisitions, net of cash acquired |
( |
) | ( |
) | ||||||||
| Net cash provided by investing activities |
||||||||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from issuance of common stock |
||||||||||||
| Principal payments on debt |
( |
) | ||||||||||
| Payments of finance leases |
( |
) | ( |
) | ( |
) | ||||||
| Payment for contingent consideration related to acquisition |
( |
) | ||||||||||
| Withholding tax payments on net share settlements on equity awards |
( |
) | ||||||||||
| Share repurchases |
( |
) | ( |
) | ||||||||
| Common stock dividends |
( |
) | ||||||||||
| Net cash used in financing activities |
( |
) | ( |
) | ( |
) | ||||||
| Effects of exchange rate changes on cash and cash equivalents |
( |
) | ||||||||||
| Net (decrease) increase in cash, cash equivalents and restricted cash |
( |
) | ( |
) | ||||||||
| Cash, cash equivalents and restricted cash, beginning of period |
||||||||||||
| Cash, cash equivalents and restricted cash, end of period |
$ | $ | $ | |||||||||
| Supplemental disclosures: |
||||||||||||
| Cash paid for interest |
$ | $ | $ | |||||||||
| Cash paid for income taxes, net |
||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses |
||||||||||||
| Reconciliation of cash, cash equivalents, and restricted cash to the consolidated balance sheets |
||||||||||||
| Cash and cash equivalents of continuing operations |
$ | $ | ||||||||||
| Short-term restricted cash included in prepaid expenses and other current assets |
||||||||||||
| Long-term restricted cash included in other assets |
||||||||||||
| Total cash, cash equivalents and restricted cash shown in the consolidated statements of cash flows |
$ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands, except share and per share data)
| Common | Accumulated | |||||||||||||||||||||||||||
| Common | Stock at | Additional | Other | Total | ||||||||||||||||||||||||
| Stock | Par | Paid-In | Comprehensive | Retained | Treasury | Stockholders’ | ||||||||||||||||||||||
| Shares | Value | Capital | Income (Loss) | Earnings | Stock | Equity | ||||||||||||||||||||||
| Balance September 30, 2021 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||
| Shares issued under restricted stock and purchase plans, net of shares withheld for employee taxes | ||||||||||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||||||
| Common stock dividends declared, at $ per share | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Unrealized gain on derivative asset, net of tax | — | |||||||||||||||||||||||||||
| Foreign currency translation adjustments reclassed out of accumulated other comprehensive income related to discontinued operations | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustments | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Changes in unrealized losses on marketable securities, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Actuarial gain on pension plans, net of tax | — | |||||||||||||||||||||||||||
| Net income | — | |||||||||||||||||||||||||||
| Other | — | |||||||||||||||||||||||||||
| Balance September 30, 2022 | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||||||||
| Shares issued under restricted stock and purchase plans, net of shares withheld for employee taxes | ( | ) | ( | ) | ||||||||||||||||||||||||
| Accelerated share repurchase | ( | ) | — | ( | ) | ( | ) | |||||||||||||||||||||
| Open market repurchases | ( | ) | — | ( | ) | ( | ) | |||||||||||||||||||||
| Retirement of treasury shares | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||||||
| Net investment hedge currency translation adjustment, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustments | — | |||||||||||||||||||||||||||
| Changes in unrealized losses on marketable securities, net of tax | — | |||||||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Actuarial gain on pension plans, net of tax | — | |||||||||||||||||||||||||||
| Other | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance September 30, 2023 | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||||||||
| Shares issued under restricted stock and purchase plans, net of shares withheld for employee taxes | ||||||||||||||||||||||||||||
| Open market repurchases | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
| Retirement of treasury shares | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Stock-based compensation | — | |||||||||||||||||||||||||||
| Net investment hedge currency translation adjustment, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustments | — | |||||||||||||||||||||||||||
| Changes in unrealized losses on marketable securities, net of tax | — | |||||||||||||||||||||||||||
| Net loss | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Actuarial loss on pension plans, net of tax | — | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance September 30, 2024 | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Operations
Azenta, Inc. (“Azenta”, or the “Company”) is a leading global provider of biological and chemical compound sample exploration and management solutions for the life sciences industry. The Company entered the life sciences market in 2011, leveraging its in-house precision automation and cryogenics capabilities that it was then applying in the semiconductor manufacturing market. This led the Company to develop and provide solutions for automated ultra-cold storage. Since then, the Company has expanded its life sciences offerings through internal investments and through a series of acquisitions. The Company now supports its customers from research and clinical development to commercialization with its sample management, automated storage, vaccine cold storage and transport, as well as genomic services expertise to help its customers bring impactful and breakthrough therapies to market faster. The Company understands the importance of sample integrity and offers a broad portfolio of products and services supporting customers at every stage of the life cycle of samples, including procurement, automated storage systems, genomic services and a multitude of sample consumables, informatics and data software, along with sample repository services. The Company's expertise, global footprint, and leadership positions enable it to be a trusted global partner to pharmaceutical, biotechnology, and life sciences research institutions.
Discontinued Operations
In the fourth quarter of fiscal year 2021, the Company entered into a definitive agreement to sell its semiconductor automation business to Thomas H. Lee Partners, L.P. (“THL”). The Company determined that the semiconductor automation business met the “held for sale” criteria and the “discontinued operations” criteria in accordance with Financial Accounting Standard Boards (“FASB”) Accounting Standards Codification (“ASC”) 205, Presentation of Financial Statements, (“FASB ASC 205”) as of September 30, 2021. Please refer to Note 3, Discontinued Operations for further information about the discontinued businesses. The Consolidated Financial Statements were restated for all periods presented to reflect the discontinuation of the semiconductor automation business and the semiconductor cryogenics business in accordance with FASB ASC 205. The discussion in the notes to these Consolidated Financial Statements, unless otherwise noted, relate solely to the Company's continuing operations.
On February 1, 2022, the Company completed the sale of the semiconductor automation business for $
2. Summary of Significant Accounting Policies
Principles of Consolidation and Basis of Presentation
The accompanying Consolidated Financial Statements include the accounts of the Company and all entities where it has a controlling financial interest and have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). All intercompany balances and transactions have been eliminated in consolidation.
Revisions to Previously Issued Financial Statements and Financial Information
In connection with the preparation of its fiscal year 2024 financial statements, the Company identified classification errors in its Consolidated Statement of Cash Flows for the year ended September 30, 2023 and the Condensed Consolidated Statements of Cash Flows for the interim periods ended March 31, 2023, June 30, 2023, December 31, 2023, March 31, 2024, and June 30, 2024. Specifically, the Company’s historical classification of the effects of exchange rate changes on the Company’s foreign denominated cash and cash equivalent balances was misclassified between the effects of exchange rate changes on cash and cash equivalents and cash flows from operating activities in its Consolidated Statement of Cash Flows for the year ended September 30, 2023 and its Condensed Consolidated Statements of Cash Flows for the interim periods ended June 30, 2023, December 31, 2023, March 31, 2024, and June 30, 2024. Additionally, the Company corrected for immaterial classification errors in between cash flows from operating activities, investing activities and financing activities, and supplemental disclosures for all revised periods. The Company's Condensed Consolidated Statements of Cash Flows for the interim periods ended March 31, 2023, June 30, 2023, December 31, 2023, March 31, 2024, and June 30, 2024 have been revised and disclosed in Note 21, Revision of Previously Issued Unaudited Quarterly Information below.
The Company assessed the effect of the errors on prior periods under the guidance of Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin No. 99, “Materiality,” codified in ASC 250, Accounting Changes and Error Corrections (“ASC 250”). Based on its assessment, the Company determined that the error correction is not material to any previously issued financial statements. The correction has no impact on the Company's previously reported consolidated net income, financial position, net change in cash, cash equivalents, and restricted cash, or total cash, cash equivalents, and restricted cash as previously reported on the Company's Consolidated Statements of Cash Flows.
Revisions of Previously Issued Audited Financial Statements
As discussed above, the identified errors did not have a material impact on the previously issued financial statements for the year ended September 30, 2023. The Company has determined it appropriate to revise the following financial statement line items in the Consolidated Statement of Cash Flows for the year ended September 30, 2023 (in thousands):
| Year ended September 30, 2023 | ||||||||||||
| As Reported | Adjustments | As Revised | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Accrued compensation and tax withholdings | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Other current assets and liabilities | ( | ) | ( | ) | ( | ) | ||||||
| Net cash provided by operating activities | $ | $ | ( | ) | $ | |||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of common stock | $ | $ | $ | |||||||||
| Net cash used in financing activities | $ | ( | ) | $ | $ | ( | ) | |||||
| Effects of exchange rate changes on cash and cash equivalents | $ | $ | $ | |||||||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
The Company also added a supplemental disclosure of purchases of property, plant and equipment included in accounts payable and accrued expenses in the amount of $
Use of Estimates
The preparation of financial statements in accordance with GAAP requires management to make certain estimates and assumptions that affect amounts reported in the financial statements and notes thereto. Although these estimates are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may differ from these estimates. Estimates are associated with recording accounts receivable, inventories, goodwill, intangible assets other than goodwill, contingent consideration liabilities related to business combinations, long-lived assets, derivative financial instruments, deferred income taxes, warranty obligations, revenue over time, stock-based compensation expense, and other accounts. The Company assesses the estimates on an ongoing basis and records changes in estimates in the period they occur and become known.
Business Combinations
The Company accounts for business acquisitions using the purchase method of accounting, in accordance with which, assets acquired (including identifiable intangible assets), and liabilities assumed are recorded at their respective fair values at the acquisition date. The fair value of the consideration paid, including contingent consideration, is assigned to the assets acquired and liabilities assumed based on their respective fair values. Goodwill represents the excess of the purchase price over the estimated fair values of the assets acquired and liabilities assumed.
Significant judgment is used in determining fair values of assets acquired and liabilities assumed and contingent consideration, as well as identified intangible assets and their estimated useful lives. Fair value and useful life determinations may be based on valuations that utilize among other factors, estimates of revenue growth rates, operating expenses, integration costs, obsolescence factors, future expected cash flows and discount rates attributable to completed technology and other acquired intangible assets. When estimating the assumptions to be used in the valuation, the Company includes a consideration of current industry information, market and economic trends, historical results of the acquired business, and other relevant factors. These assumptions are forward-looking and could be affected by future economic and market conditions. Adjustments to fair values of assets and liabilities made after the end of the measurement period are recorded within our operating results.
Changes in the fair value of contingent consideration resulting from a change in the underlying inputs are recognized in results of operations until the arrangement is settled.
Foreign Currency Translation and Transaction Accounting
All assets and liabilities of the Company’s subsidiaries operating in non-U.S. dollar currencies are translated into the reporting currency at period-end exchange rates, while revenue, expenses, gains and losses are translated at the average exchange rates during the period. Resulting translation adjustments are reflected in the “Accumulated other comprehensive income (loss)” in the Company’s Consolidated Balance Sheets and presented as “Foreign currency translation adjustments” in the Company’s Consolidated Statements of Comprehensive Income (Loss).
The determination of the functional currency of the Company’s subsidiaries is based on their financial and operational environment and is the local currency of the Company’s foreign subsidiaries. Certain transactions of the Company and its subsidiaries are denominated in currencies other than their functional currency. Foreign currency exchange gains (losses) generated from the settlement and remeasurement of these transactions are recognized in earnings and presented within “Other income (expense)” in the Company’s Consolidated Statements of Operations. Net foreign currency transaction and remeasurement losses totaled $
The semiconductor automation business had foreign operations which had a cumulative translation adjustment balance of $
Derivative Financial Instruments
The Company has transactions and balances denominated in currencies other than the functional currency of the transacting entity. Most of these transactions carry foreign exchange risk in Germany, the United Kingdom and China. The Company enters into foreign exchange contracts to reduce its exposure to currency fluctuations. The arrangements typically mature in three months or less and they do not qualify for hedge accounting. Net gains and losses related to foreign exchange contracts are recorded as a component of “Other income (expense)” in the accompanying Consolidated Statements of Operations.
The fair values of the forward contracts are recorded in the accompanying Consolidated Balance Sheets as “Prepaid expenses and other current assets” and “Accrued expenses and other current liabilities”. Foreign exchange contract assets and liabilities are measured and reported at fair value based on observable market inputs and classified within Level 2 of the fair value hierarchy described below due to a lack of an active market for these contracts.
All derivatives, whether designated as a hedging relationship or not, are recorded in the Consolidated Balance Sheets at fair value. The accounting for changes in fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, the Company must designate the hedging instrument as a fair value hedge, cash flow hedge or a hedge of a net investment in a foreign operation based on the exposure being hedged. Certain derivatives held by the Company are not designated as hedges but are used in managing exposure to changes in foreign exchange rates.
A fair value hedge is a derivative instrument designated for the purpose of hedging the exposure to changes in fair value of an asset or a liability resulting from a particular risk. If the derivative is designated as a fair value hedge, the changes in the fair value of the derivative and of the hedged item attributable to the hedged risk are both recognized in the results of operations and presented in the same caption in the Consolidated Statements of Operations and Consolidated Statements of Comprehensive Income.
A cash flow hedge is a derivative instrument designated for the purpose of hedging the exposure to variability in future cash flows resulting from a particular risk. If the derivative is designated as a cash flow hedge, the effective portions of changes in the fair value of the derivative are recorded in “Accumulated other comprehensive income (loss)” in the Consolidated Balance Sheets and recognized in the results of operations when the hedged item affects earnings. Ineffective portions of changes in the fair value of cash flow hedges are recognized in the results of operations.
A hedge of a net investment in a foreign operation is achieved through a derivative instrument designated for the purpose of hedging the exposure of changes in value of investments in foreign subsidiaries. If the derivative is designated as a hedge of a net investment in a foreign operation, the effective portions of changes in the fair value of the derivative are recorded in “Accumulated other comprehensive income (loss)” as a part of the foreign currency translation adjustment. Ineffective portions of net investment hedges are recognized in the results of operations.
For derivative instruments not designated as hedging instruments, changes in fair value are recognized in the Consolidated Statements of Operations as gains or losses consistent with the classification of the underlying risk.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash deposits and cash equivalents, marketable securities, derivative instruments, and accounts receivable. All of the Company’s cash and cash equivalents, restricted cash, marketable securities and derivative instruments are maintained by major financial institutions.
The Company invests cash not used in operations in investment grade, high credit quality securities in accordance with the Company’s investment policy which provides guidelines and limits regarding investments type, concentration, credit quality and maturity terms aimed at maintaining liquidity and reducing risk of capital loss.
The Company regularly monitors the creditworthiness of its customers and believes that it has adequately provided for exposure to potential credit losses. The Company’s ten largest customers accounted for approximately
Marketable Securities
The Company invests in marketable securities that are classified as available-for-sale and records them at fair value in the Consolidated Balance Sheets. Marketable securities reported as current assets represent investments that mature within one year from the balance sheet date. Long-term marketable securities represent investments with maturity dates greater than one year from the balance sheet date.
Unrealized gains and losses are excluded from earnings and reported as a separate component of “Accumulated other comprehensive income (loss)” in the Consolidated Balance Sheets until the security is sold or matures. Gains or losses realized from sales of marketable securities are computed based on the specific identification method and recognized as a component of “Other income (expense)” in the accompanying Consolidated Statements of Operations.
The Company reviews the marketable securities for impairment at each reporting date to determine if any of the securities have experienced an other-than-temporary decline in fair value. The Company considers factors, such as the length of time and extent to which the market value has been less than the cost, the financial condition and near-term prospects of the issuer, the Company’s intent to sell, or whether it is more likely than not it will be required to sell the investment before recovery of its amortized cost basis. If the Company believes that an other-than-temporary decline in fair value has occurred, it writes down the investment to its fair value and recognizes the credit loss in earnings and the non-credit loss in “Accumulated other comprehensive income (loss)” in the Consolidated Balance Sheets.
Fair Value Measurement
The Company measures certain financial assets and liabilities, including cash equivalents, available-for-sale securities, accounts receivable, accounts payable, contingent consideration liability, and derivative instruments at fair value. The Company applies a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The following levels of inputs may be used to measure fair value:
| ● | Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities that are accessible as of the reporting date. Active markets are those in which transactions for the asset and liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis. The fair value hierarchy gives the highest priority to Level 1 inputs. |
| ● | Level 2: Observable inputs other than prices included in Level 1, including quoted prices for similar assets or liabilities in active markets and quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| ● | Level 3: Unobservable inputs that are significant to the fair value of the assets or liabilities and reflect an entity’s own assumptions in pricing assets or liabilities since they are supported by little or no market activity. |
In determining fair value, the Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs, as well as considering counterparty credit risk in its assessment of fair value.
The Company measures certain assets, including the cost and equity method investments, at fair value on a nonrecurring basis when they are deemed to be other-than-temporarily impaired. The fair values of these investments are determined based on valuation techniques using the best information available, and may include quoted market prices, market comparable prices, and discounted cash flow projections. An impairment charge is recorded when the cost of the investment exceeds its fair value and this condition is determined to be other-than-temporary
Cash and Cash Equivalents, and Restricted Cash
Cash and cash equivalents consist of cash and highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash. Cash equivalents are reported at fair value.
The Company classifies long-term restricted cash balances within “Other assets” on the accompanying Consolidated Balance Sheets based upon the term of the remaining restrictions.
Accounts Receivable and Allowance for Expected Credit Losses
Trade accounts receivable do not bear interest and are recorded at the invoiced amount. The Company maintains an allowance for expected credit losses representing its best estimate of expected credit losses related to its existing accounts receivable and their net realizable value. The Company determines the allowance based on several factors, including an evaluation of customer credit worthiness, the age of the outstanding receivables, economic trends, historical experience and other information over the payment periods. The Company reviews and adjusts the allowance for expected credit losses on a quarterly basis. Accounts receivable balances are written off against the allowance for expected credit losses when the Company determines that the balances are not recoverable. Provisions for expected credit losses are recorded in “Selling, general and administrative” expenses in the Consolidated Statements of Operations. The Company does not have any off-balance-sheet credit exposure related to its customers.
Inventories
Inventories are stated at the lower of cost or net realizable value determined on a first-in, first-out basis and include the cost of materials, labor and manufacturing overhead. The Company reports inventories at their net realizable value and provides reserves for excess, obsolete or damaged inventory based on changes in customer demand, technology and other economic factors.
Fixed Assets, Intangible Assets and Impairment of Long-lived Assets
Property, plant and equipment are stated at cost, net of accumulated depreciation. Depreciation expense is computed based on the straight-line method and charged to results of operations to allocate the cost of the assets over their estimated useful lives, as follows:
| Buildings | ||||
| Computer equipment and software | ||||
| Machinery and equipment | ||||
| Furniture and fixtures | 5 years | |||
| Vehicles |
Leasehold improvements are amortized over the shorter of their estimated useful lives or the remaining terms of the respective leases. Equipment used for demonstrations to customers is included in machinery and equipment and depreciated over its estimated useful life. Repair and maintenance costs are expensed as incurred.
The Company has developed software for internal use. Internal and external labor costs incurred during the application development stage of a project are capitalized. Costs incurred prior to application development and post implementation are expensed as incurred. Training and data conversion costs are expensed as incurred.
Long lived assets and their associated accumulated depreciation are derecognized upon their retirement or at the time of disposal, and the resulting gain or loss is included in the Company’s results of operations.
The Company identified finite-lived intangible assets other than goodwill as a result of acquisitions. Finite-lived intangible assets are valued based on estimated future cash flows and amortized over their estimated useful lives based on methods that approximate the pattern in which the economic benefits are expected to be realized.
Finite-lived intangibles assets and fixed assets are tested for impairment when indicators of impairment are present. For purposes of this test, long-lived assets are grouped with other assets and liabilities at the lowest level for which identifiable cash flows are largely independent of the cash flows of other assets and liabilities. If the Company determines that indicators of potential impairment are present, it assesses the recoverability of long-lived asset group by comparing its undiscounted future cash flows to its carrying value, and an impairment loss is recognized in operating results to the extent any finite-lived intangible asset’s carrying value exceeds its calculated fair value. The future cash flow period is based on the future service life of the primary asset within the long-lived asset group.
Finite-lived intangible assets are amortized over their useful lives, as follows:
| Trademarks | 13 years | |||
| Patents | 8 years | |||
| Completed technology | ||||
| Customer relationships |
Leases
The Company has operating leases for real estate and non-real estate and finance leases for non-real estate. The classification of a lease as operating or finance and the determination of the right-of-use asset (“ROU asset”) and lease liability are determined at lease inception. The ROU asset represents the Company’s right to use an underlying asset for the lease term and the lease liability represents the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at the commencement date of the lease based on the present value of lease payments over the lease term. As most of the Company’s leases do not provide an implicit rate, an incremental borrowing rate is used based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. Lease terms may include options to extend or terminate the lease when it is reasonably certain that the option will be exercised. Lease expense is recognized on a straight-line basis over the lease term.
The Company’s lease agreements may contain lease and non-lease components. Non-lease components primarily include payments for maintenance and utilities. Fixed payments for non-lease components are combined with lease payments and accounted for as a single lease component which increases the amount of the ROU asset and liability.
The ROU asset for operating leases is included within “Operating lease right-of-use assets” and the ROU asset for finance leases is included within “Property, plant and equipment, net” in the Consolidated Balance Sheets. The short-term lease liabilities for both operating leases and finance leases are included within “Accrued expenses and other current liabilities” in the Consolidated Balance Sheets. The long-term lease liabilities for operating leases and finance leases are included within “Long-term operating lease liabilities”, and “Other long-term liabilities”, respectively, in the Consolidated Balance Sheets.
Goodwill
Goodwill represents the excess of purchase price over the fair value of net tangible and identifiable intangible assets of businesses acquired by the Company. Goodwill is tested for impairment annually or more often if impairment indicators are present at the reporting unit level. The Company elected April 1st as its annual goodwill impairment assessment date. If the existence of events or circumstances indicates that it is more likely than not that fair values of the reporting units are below their carrying values, the Company performs additional impairment tests during interim periods to evaluate goodwill for impairment.
Application of the goodwill impairment test requires significant judgment based on market and operational conditions at the time of the evaluation, including management’s best estimate of future business activity and the related estimates of future cash flows from the reporting units that include the associated goodwill. These periodic evaluations could cause management to conclude that impairment factors exist, requiring an adjustment of these assets to their then-current fair market values. Future business conditions and/or activity could differ materially from the projections made by management which could result in impairment charges.
The goodwill impairment test is performed at the reporting unit level. A reporting unit is either an operating segment or one level below it, which is referred to as a “component”. The level at which the impairment test is performed requires an assessment of whether the operations below an operating segment constitute a self-sustaining business, in which case testing is generally performed at this level.
The Company first assesses qualitative factors to determine whether the existence of events or circumstances indicates that it is more likely than not that the fair value of a reporting unit is less than its carrying value. If the Company determines, based on this assessment, that it is more likely than not that the fair value of the reporting unit is less than its carrying value, management performs a quantitative goodwill impairment test by comparing the reporting unit’s fair value with its carrying value. An impairment loss is recognized for the amount by which the reporting unit’s carrying value exceeds its fair value, up to the total amount of goodwill allocated to the reporting unit.
We determine fair values of our reporting units based on an income approach in accordance with the discounted cash flow method, (the “DCF Method”). The DCF Method is based on projected future cash flows and terminal value estimates discounted to their present values. Terminal value represents the present value an investor would pay on the valuation date for the rights to the cash flows of the business for the years subsequent to the discrete cash flow projection period. We consider the DCF Method to be the most appropriate valuation technique since it is based on management’s long-term financial projections. In addition to determining the fair value of our reporting units based on the DCF Method, we also compare the aggregate values of our net corporate assets and reporting unit fair values to our overall market capitalization and use certain market-based valuation techniques to assess the reasonableness of the reporting unit fair values determined in accordance with the DCF Method.
Warranty Obligations
The Company establishes reserves for estimated costs of product warranties based on historical information. Product warranty reserves are recorded at the time product revenue is recognized, and retrofit accruals are recorded at the time retrofit programs are established. The Company’s warranty obligation is affected by product failure rates, utilization levels, material usage, service delivery costs incurred in correcting a product failure and supplier warranties on parts delivered by the Company.
Revenue Recognition
The Company generates revenue from the following sources:
| ● | Products, including sales of automated cold sample management systems, vaccine cold storage and transport systems, consumables, instruments, spare parts, and software. |
| ● | Services, including repairs, upgrades, diagnostic support, installation, as well as biological sample services such as DNA sequencing, gene synthesis, molecular biology, bioinformatics, biological sample storage, sample acquisition, and other support services. |
The Company recognizes revenue for the transfer of such promised products or services to customers in an amount that reflects the consideration to which the Company expects to be entitled to in exchange for those products or services. Under ASC 606, Revenue from Contracts with Customers (“ASC 606”), revenue is recognized when, or as, the transfer of control of the underlying performance obligation occurs. To determine the amount of consideration the Company expects to be entitled to and whether transfer of control has occurred, the Company applies the following five-step model:
| ● | Identify the contract with a customer. Contracts are accounted for when approval and commitment has been received from both parties, the rights of each party are identified, payment terms are identified, the contract has commercial substance and collectability of the consideration to which the Company is entitled is probable. Contracts are generally evidenced through receipt of an approved purchase order or execution of a binding arrangement and can be both short and long-term. Long-term contracts within the segments relate to the sale of products with attached service-type warranty contracts that generally have a stated contract term that is greater than one year. Contracts may contain acceptance provisions where the Company is required to obtain technical acceptance from the customer upon completion of installation services and evidence of the system’s functional performance within the customer’s operating environment. The Company has concluded that acceptance criteria within its contracts can be objectively evaluated and will not impact the Company’s transfer of control assessment under ASC 606. |
| ● | Identify the performance obligations in the contract. Performance obligations include the sale of products and services. Certain customer arrangements related to the sale of automated cold sample management systems generally include more than one performance obligation and may include a combination of goods and/or services, such as products with installation services or service-type warranty obligations. These contracts include multiple promises and as a result, the Company is required to evaluate each promise and determine whether the promise qualifies as a performance obligation within the contract. Contracts may contain the option to acquire additional products or services at defined prices. The Company reviews the pricing of these options to determine whether the option would exist independently of the current contract. If the pricing of contract options provides a material right to the customer that it would not receive without entering into the current contract, the Company accounts for the option as a separate performance obligation. |
| ● | Determine the transaction price. The transaction price of the Company’s contracts with its customer is generally fixed, based on the amounts to be contractually billed to the customer. Although uncommon, certain contracts may contain variable consideration in the form of customer allowances and rebates that consist primarily of retrospective volume-based discounts and other incentive programs. Variable consideration is estimated at contract inception and included in the transaction price if it is probable that a subsequent change in the estimate would not result in a significant revenue reversal. The period between transfer of control of the performance obligations within a customer contract and timing of payment is generally within one year. As a result, the Company’s contracts typically do not include significant financing components.
|
| ● | Allocate the transaction price to the performance obligations in the contract. For customer contracts that contain more than one performance obligation, the Company allocates the total transaction consideration to each performance obligation based on the relative stand-alone selling price of each performance obligation within the contract. The Company relies on either observable standalone sales or an expected cost-plus margin approach to determine the standalone selling price of offerings, depending on the nature of the performance obligation. Performance obligations whose standalone selling price is estimated using an expected cost-plus margin approach relate to the sale of customized automated cold sample management systems, services, and service-type warranties. |
| ● | Recognize revenue when or as the Company satisfies a performance obligation. The Company satisfies its performance obligations by transferring a product or service either at a point in time or over time, when the transfer of control of the underlying performance obligation has occurred. Control is evidenced by the customer’s ability to direct the use of and obtain substantially all the remaining benefits from the performance obligation. Revenue from third-party sales for which the Company does not meet the criteria for gross revenue recognition is recognized on a net basis. All other revenue is recognized on a gross basis. The Company excludes from the transaction price all sales taxes assessed by governmental authorities and as a result, revenue is presented net of tax. |
As a result of applying this five-step model under ASC 606, the Company recognizes revenues from its sale of products and services as follows:
| ● | Products: Revenue from the sale of standard products is recognized upon their transfer of control to the customer, which is considered complete at either the time of shipment or arrival at destination, based on the agreed upon terms within the contract. The Company’s payment terms for the sale of standard products are typically | |
| Revenue from the sales of certain products that involve significant customization, which include primarily automated cold sample management systems is recognized over time as the asset created by the Company’s performance does not have alternative use to the Company and an enforceable right to payment for performance completed to date is present. The Company recognizes revenue as work progresses based on a percentage of actual labor hours incurred on the project to-date and total estimated labor hours expected to be incurred on the project. The selection of the method to measure progress towards completion requires judgment. The Company has concluded that using the percentage of labor hours incurred to estimated labor hours needed to complete the project most appropriately depicts the Company’s efforts towards satisfaction of the performance obligation. The Company develops profit estimates for long-term contracts based on total revenue expected to be generated from the project and total costs anticipated to be incurred in the project. These estimates are based on a number of factors, including the degree of required product customization and the work required to be able to install the product in the customer’s existing environment, as well as the Company’s historical experience, project plans and an assessment of the risks and uncertainties inherent in the contract related to implementation delays or performance issues that may or may not be within the Company’s control. The Company estimates a loss on a contract by comparing total estimated contract revenue to the total estimated contract costs and recognizes a loss during the period in which it becomes probable and can be reasonably estimated. The Company reviews profit estimates for long-term contracts during each reporting period and revises the estimate based on changes in circumstances. Revenue for certain arrangements that involve significant product customization but do not provide the Company with an enforceable right to payment for performance completed to date are recognized at a point in time, upon completion or substantial completion of the project, provided transfer of control has occurred. The project is considered substantially complete when the Company receives acceptance from the customer and remaining tasks are perfunctory or inconsequential and in control of the Company. Generally, the terms of long-term contracts provide for progress billings based on completion of milestones or other defined phases of work. In certain instances, payments collected from customers in advance of recognizing the related revenue are recorded and presented as contract liabilities within “Deferred revenue” on the Company’s Consolidated Balance Sheet. Additionally, due to certain billing constraints within contracts, the customer may retain a portion of the contract price until completion of the contract. In these contracts, an unbilled receivable is recorded when revenue recognized may exceed billings, which the Company presents as a contract asset on the balance sheet, which is included within the “Prepaid expenses and other current assets” on the Company’s Consolidated Balance Sheet. |
| ● | Services: Service revenue is generally recognized ratably over time or on an output method, as the customer simultaneously receives and consumes the benefit of these services as they are performed. Payments related to service-type warranties may be made up front or proportionally over the contract term. Revenue for sample management and storage are recognized over the period the services are rendered or samples are stored. Revenue from genomic services is recognized over time and is based upon the fact that transfer of control takes place over time as determined using the input method of costs incurred. Payment due or received from the customers prior to rendering the associated services are recorded as a contract liability. |
Government Assistance
The Company receives government assistance from various domestic and foreign, local, regional and national governments which vary in size and duration in the form of cash grants or refundable tax credits. The government assistance typically specifies conditions that must be met in order for it to be earned, such as employee retention targets, completion of employee training, or the construction or acquisition of property and equipment and are often time bound. If conditions are not satisfied or if the duration period for the arrangement is not met, the government assistance is often subject to reduction, repayment, or termination.
The Company’s policy is to recognize a benefit in the Consolidated Statements of Operations in “” over the life of the asset or duration of the program when the Company has reasonable assurance that it will comply with the conditions under the government assistance and the government assistance will be received, refundable tax credits may also result in a reduction in “Accrued income taxes payable” in the Consolidated Balance Sheets. If government assistance is received or is probable of receipt and the amount is determinable by the Company in advance of completion of the conditions, the government assistance is recognized in “Accrued expenses and other current liabilities” or “Other long-term liabilities” in the Consolidated Balance Sheets, as appropriate.
In fiscal year 2024, approximately $
Research and Development Expense
Research and development costs are expensed as incurred. Research and development costs consist primarily of personnel expenses related to development of new products, as well as enhancements and engineering changes to existing products and development of hardware and software components.
Restructuring Charges
Accounting for the timing and amount of termination benefits provided by the Company to employees is determined based on whether: (a) the Company has a substantive plan to provide such benefits, (b) the Company has a written employment contract with the affected employees that includes a provision for such benefits, (c) the termination benefits are due to the occurrence of an event specified in an existing plan or agreement, or (d) the termination benefits are a one-time benefit. In certain circumstances, employee termination benefits may meet more than one of the characteristics listed above and therefore, may have individual elements that are subject to different accounting models.
From time to time when executing a restructuring or exit plan, the Company also incurs costs other than termination benefits, such as impairments of long-lived assts that are no longer used in operations (including right-of-use assets, facility-related property and equipment) and termination costs or penalties to cancel a contractual obligation. Such costs are recognized when incurred, which generally occurs at the contract termination or over the period from when a plan to abandon a leased facility is approved through the cease-use date but charges may continue over the remainder of the original contractual period.
Stock-Based Compensation
The fair value of restricted stock units is determined based on the number of shares granted and the closing price of the Company’s common stock quoted on the Nasdaq Stock Market on the date of grant. For awards that vest based on service conditions, the Company recognizes stock-based compensation expense on a straight-line basis over the requisite service period. For awards that vest subject to performance conditions, the Company recognizes stock-based compensation expense ratably over the performance period if it is probable that performance condition will be met and adjusts for the percentage of shares probable of achieving the performance goals. Each quarter, management assesses the probability of achieving the performance goals. The Company makes estimates of stock award forfeitures and the number of awards expected to vest. The Company considers many factors in developing forfeiture estimates, including award types, employee classes and historical experience. Current estimates may differ from actual results and future changes in estimates.
Income Taxes
The Company records income taxes using the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax differences between the financial statement carrying amounts of existing assets and liabilities and their respective income tax bases, as well as operating loss and tax credit carryforwards. The Company’s Consolidated Financial Statements contain certain deferred tax assets that were recorded as a result of operating losses, as well as other temporary differences between financial and tax accounting. A valuation allowance is established against deferred tax assets if, based upon the evaluation of positive and negative evidence and the extent to which that evidence is objectively verifiable, it is more likely than not that some or all of the deferred tax assets will not be realized.
Significant management judgment is required in determining the Company’s income tax (benefit) expense, the Company’s deferred tax assets and liabilities and any valuation allowance recorded against those net deferred tax assets. The Company evaluates the weight of all available evidence to determine whether it is more likely than not that some portion or all of the net deferred income tax assets will not be realized.
The calculation of the Company’s income tax liabilities involves consideration of uncertainties in the application of complex tax regulations. The Company recognizes liabilities for uncertain tax positions based on a two-step process. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon an audit conducted by taxing authorities, including resolution of related appeals or litigation processes, if any. If the Company determines that a tax position will more likely than not be sustained, the second step requires the Company to estimate and measure the tax benefit as the largest amount that is more likely than not to be realized upon ultimate settlement. It is inherently difficult and subjective to estimate such amounts, as the Company must determine the probability of various possible outcomes. The Company re-evaluates these uncertain tax positions on a quarterly basis. This evaluation is based on factors, such as changes in facts or circumstances, tax law, new audit activity and effectively settled issues. Determining whether an uncertain tax position is effectively settled requires judgment. A change in recognition or measurement may result in the recognition of a tax benefit or an additional charge to the tax expense.
Net Income (Loss) per Share
Basic income or loss per share is determined by dividing net income or loss by the weighted average common shares outstanding during the period. Diluted income or loss per share is determined by dividing net income or loss by diluted weighted average shares outstanding during the period. Diluted weighted average shares reflect the dilutive effect, if any, of potential common shares. To the extent their effect is dilutive, employee equity awards and other commitments to be settled in common stock are included in the calculation of diluted income or loss per share based on the treasury stock method. Potential common shares are excluded from the calculation of dilutive weighted average shares outstanding if their effect would be anti-dilutive at the balance sheet date based on a treasury stock method or due to a net loss.
Recently Adopted Accounting Pronouncements
In October 2023, the FASB issued Accounting Standards Update (“ASU”) 2023-06, Disclosure Improvements: Codification Amendments in Response to the SEC’s Disclosure Update and Simplification Initiative. The ASU aligns the requirements in FASB’s ASC with SEC regulations. The effective date for each amendment is the date on which the SEC removal of the related disclosure requirement from Regulation S-X or Regulation S-K becomes effective, or if the SEC does not remove the requirement by June 30, 2027, the amendment will not become effective for any entity. Early adoption is prohibited. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements or disclosures.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. The ASU requires the disclosure of incremental segment information on an annual and interim basis, primarily through enhanced disclosures pertaining to significant segment expenses. This update is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, and requires retrospective application to all prior periods presented in the financial statements. The Company is currently evaluating the standard to determine the impact of adoption on its disclosures. The Company does not expect that the standard will have an impact on its consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. The ASU is intended to enhance the transparency and decision usefulness of income tax disclosures primarily through changes to the rate reconciliation and income taxes paid information. This update is effective for annual periods beginning after December 15, 2024, though early adoption is permitted. The Company does not expect the adoption of this standard to impact its consolidated financial statements until fiscal year 2026.
In March 2024, the SEC issued final rules under SEC Release No. 33-11275, The Enhancement and Standardization of Climate-Related Disclosures for Investors. Effective fiscal year 2026, the Company is required to disclose climate-related risks that are reasonably likely to have a material impact on the Company’s business strategy, results of operations, or financial condition. Additionally, the Company will be required to disclose the effects of severe weather events and other natural conditions within the notes to the financial statements, subject to certain materiality thresholds. Effective fiscal year 2027, required disclosures will also include disclosure of material direct greenhouse gas emissions from operations owned or controlled (Scope 1) and material indirect greenhouse gas emissions from purchased energy consumed in owned or controlled operations (Scope 2). In April 2024, the SEC issued an order voluntarily staying the effectiveness of the new rules pending the completion of judicial review of certain legal challenges to their validity. The Company is currently evaluating the impact of these rules assuming adoption as well as monitoring the status of the related litigation and the SEC’s stay which remains in effect as of September 30, 2024.
In 2021, the Organization of Economic Cooperation and Development (“OECD”) introduced its Pillar II Framework Model Rules (“Pillar 2”), which are designed to impose a 15% global minimum tax on the earnings of in-scope multinational corporations on a country-by-country basis. Certain aspects of Pillar 2 took effect on January 1, 2024 while other aspects go into effect on January 1, 2025. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements as the Company does not expect to meet the consolidated revenue threshold of €750 million over the next twelve months.
3. Discontinued Operations
Disposition of the Semiconductor Automation Business
On September 20, 2021, the Company entered into a definitive agreement to sell its semiconductor automation business to Thomas H. Lee Partners, L.P. (“THL”) and the Company determined that the semiconductor automation business met the criteria to be classified as a discontinued operation and, as a result, its historical financial results are reflected in the consolidated financial statements as a discontinued operation, and assets and liabilities were classified as assets and liabilities held for sale. On February 1, 2022, the Company completed the sale of the semiconductor automation business for $
In connection with the closing of the sale, the Company and THL entered into a transition services agreement under which both the Company and THL provide each other certain transition services related to finance and accounting, information technology, human resources, compliance, facilities, legal and research and development support, for time periods which ranged from to
During the twelve months ended September 30, 2023, the Company recorded a $
| Year Ended September 30, | ||||
| 2022 | ||||
| Revenue | ||||
| Products | $ | |||
| Services | ||||
| Total revenue | ||||
| Cost of revenue | ||||
| Products | ||||
| Services | ||||
| Total cost of revenue | ||||
| Gross profit | ||||
| Operating expenses | ||||
| Research and development | ||||
| Selling, general and administrative | ||||
| Restructuring charges | ||||
| Total operating expenses | ||||
| Operating income | ||||
| Gain on divestiture | ||||
| Income before income taxes | ||||
| Income tax expense | ||||
| Net income from discontinued operations | $ | |||
The following table presents the significant non-cash items and capital expenditures for the discontinued operations with respect to the semiconductor automation business that are included in the Consolidated Statements of Cash Flows (in thousands):
| Year Ended September 30, | ||||
| 2022 | ||||
| Capital expenditures | ||||
There were no significant non-cash items and or capital expenditures related to discontinued operations during the fiscal years ended September 30, 2024 and 2023.
4. Business Combinations
The Company recorded the assets acquired and liabilities assumed related to the following acquisitions at their fair values as of the acquisition date, from a market participant’s perspective. While the Company uses its best estimates and assumptions as part of the purchase price allocation process to value the assets acquired and liabilities assumed on the acquisition date, its estimates and assumptions are subject to refinement. Fair value estimates are based on a complex series of judgments about future events and uncertainties and rely heavily on estimates and assumptions. The judgments used to determine the estimated fair value assigned to each class of assets acquired and liabilities assumed, as well as asset lives, can materially impact the Company’s results of operations. The measurement period to finalize the fair values is completed within one year after the respective acquisition date.
Acquisitions Completed in Fiscal Year 2023
Ziath Ltd
On February 2, 2023, the Company acquired Ziath, Ltd. and its subsidiaries (“Ziath”). Based in Cambridge, United Kingdom, Ziath is a leading provider of 2D barcode readers for life science applications. Founded in 2005, Ziath’s innovative 2D barcode readers are a key component of the laboratory automation workflow serving pharmaceutical, biotechnology and academic customers worldwide. Ziath is expected to enhance the Company’s offerings, which support the entire lifecycle of sample management from specimen collection to sample registration, storage and processing. The acquisition was completed at a purchase price of $
The allocation of the consideration included $
The Company did not present pro forma financial information for its consolidated results of operations for the acquisition because such results are immaterial.
B Medical Systems S.á. r.l.
On October 3, 2022, the Company acquired B Medical Systems S.á r.l. and its subsidiaries ("B Medical"), for a purchase price of $
The contingent consideration payment from the Company to the Seller was based on achievement of certain revenue targets over the -year period from October 3, 2022 to September 30, 2023. The Company recorded the estimated fair value of the contingent consideration liability utilizing a Monte Carlo simulation that incorporates revenue projections, revenue growth rates of comparable companies, implied volatility and a risk adjusted discount rate. Each quarter, the Company was required to remeasure the fair value of this liability as assumptions changed over time and any resulting adjustments in the fair value of this liability were recorded in “Operating expenses” in the Consolidated Statements of Operations. This fair value measurement was based on significant inputs not observable in the market and thus represented a Level 3 measurement. This fair value measurement was directly impacted by the Company’s estimate of future incremental revenue growth of the business. Accordingly, if actual revenue growth was higher or lower than the estimates within the fair value measurement, the Company would record additional charges or gains. This liability was revalued from $
The purchase price was allocated to B Medical’s tangible and identifiable intangible assets acquired and liabilities assumed based on the estimated fair values as of October 3, 2022, as set forth below (in thousands):
| Fair Value | ||||
| Accounts receivable | $ | |||
| Inventory | ||||
| Other assets | ||||
| Property plant and equipment | ||||
| Identifiable Intangible Assets: | ||||
| Completed technology | ||||
| Trademarks | ||||
| Customer relationships | ||||
| Backlog | ||||
| Other liabilities | ( | ) | ||
| Deferred income taxes, net | ( | ) | ||
| Goodwill | ||||
| Total purchase price, net of cash acquired | $ | |||
During the twelve months ended September 30, 2023 the Company recorded adjustments which resulted in a net increase to goodwill of $
In performing the purchase price allocation, the Company considered, among other factors, the intended future use of acquired assets, and historical financial performance and estimates of future performance of B Medical’s business. As part of the purchase price allocations, the Company determined the identifiable intangible assets were completed technology value, trademarks, customer relationships and backlog. The fair value of the intangible assets was estimated using the income approach, specifically the multi-period excess earnings method, and the cash flow projections were discounted using a rate of
The acquired intangible assets and goodwill are subject to review for impairment if indicators of impairment develop and otherwise at least annually. See Note 8, Goodwill and Intangible Assets below for information about the impairment of this goodwill during the second quarter of fiscal year 2024.
The following financial information reflects the Company's consolidated results from B Medical (in thousands):
| Year Ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| Revenue | $ | $ | ||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
The results of the Company’s quantitative goodwill impairment analyses as of March 31, 2024 indicated an impairment of goodwill within its B Medical Systems reporting unit resulting in a non-cash impairment charge of $
The following unaudited pro forma financial information reflects the Company's consolidated results of operations as if the acquisition had taken place on October 1, 2021 (in thousands). The unaudited pro forma financial information is not necessarily indicative of the results of operations that the Company would have reported had the transaction occurred at the beginning of these periods nor is it necessarily indicative of future results.
| Year Ended September 30, | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | $ | ||||||
| Net income (loss) | $ | ( | ) | $ | ||||
To present the Company's consolidated results of operations as if the acquisition had taken place on October 1, 2021, the unaudited pro forma earnings for the fiscal years 2023 and 2022 have been adjusted to exclude $
Acquisition Completed in Fiscal Year 2022
Barkey Holding GmbH
On July 1, 2022, the Company acquired Barkey Holding GmbH and its subsidiaries (“Barkey”), a leading provider of controlled-rate thawing devices for customers in the medical, biotechnology and pharmaceutical industries, headquartered in Leopoldshöhe, Germany. The financial results for Barkey are included within the Sample Management Solutions segment. The total cash purchase price of the acquisition was $
During the twelve months ended September 30, 2023 the Company recorded an adjustment which resulted in an increase to goodwill of $
The Company did not present pro forma financial information for its consolidated results of operations for the acquisition because such results are immaterial.
5. Marketable Securities
During fiscal years 2024 and 2023, the Company had sales and maturities of marketable securities of $
The following is a summary of the amortized cost and the fair value, including accrued interest receivable, as well as unrealized gains (losses) on the short-term and long-term marketable securities as of September 30, 2024 and 2023 (in thousands):
| Gross | Gross | |||||||||||||||
| Amortized | Unrealized | Unrealized | ||||||||||||||
| Cost | Losses | Gains | Fair Value | |||||||||||||
| September 30, 2024: | ||||||||||||||||
| U.S. Treasury securities and obligations of U.S. government agencies | $ | $ | ( | ) | $ | $ | ||||||||||
| Bank certificates of deposits | ( | ) | ||||||||||||||
| Corporate securities | ( | ) | ||||||||||||||
| $ | $ | ( | ) | $ | $ | |||||||||||
| September 30, 2023: | ||||||||||||||||
| U.S. Treasury securities and obligations of U.S. government agencies | $ | $ | ( | ) | $ | $ | ||||||||||
| Bank certificates of deposits | ( | ) | ||||||||||||||
| Corporate securities | ( | ) | ||||||||||||||
| $ | $ | ( | ) | $ | $ | |||||||||||
The fair values of the marketable securities by contractual maturities at September 30, 2024 are presented below (in thousands).
| Amortized | ||||||||
| Cost | Fair Value | |||||||
| Due in one year or less | $ | $ | ||||||
| Due after one year through five years | ||||||||
| Due after five years through ten years | ||||||||
| Due after ten years | ||||||||
| Total marketable securities | $ | $ | ||||||
Expected maturities could differ from contractual maturities because the security issuers may have the right to prepay obligations without prepayment penalties.
Unrealized losses from fixed-income securities are primarily attributable to changes in interest rates. The Company does not believe any unrealized losses represent impairments based on the evaluation of the available evidence.
6. Derivative Instruments
Net gains and losses related to foreign exchange contracts are recorded as a component of “Other income (expense)” in the accompanying Consolidated Statements of Operations and are as follows for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Realized (losses) gains on derivatives not designated as hedging instruments | $ | ( | ) | $ | ( | ) | $ | |||||
The notional amounts of the Company’s derivative instruments as of September 30, 2024 and 2023 were as follows (in thousands):
| Year Ended September 30, | |||||||||
| Hedge Designation | 2024 | 2023 | |||||||
| Cross-currency swap | Net Investment Hedge | $ | $ | ||||||
| Foreign exchange contracts | Undesignated | ||||||||
The fair value of derivative instruments are as follows at September 30, 2024 and 2023 (in thousands):
| Fair Value of Assets | Fair Value of Liabilities | |||||||||||||||
| As of September 30, | 2024 | 2023 | 2024 | 2023 | ||||||||||||
| Derivatives designated as hedging instruments | ||||||||||||||||
| Cross-currency swap | $ | $ | $ | ( | ) | $ | ||||||||||
| Derivatives not designated as hedging instruments | ||||||||||||||||
| Foreign exchange contracts | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
| Total fair value | $ | $ | $ | ( | ) | $ | ( | ) | ||||||||
Hedging Activities
On February 1, 2022, the Company entered into a cross-currency swap agreement to hedge the variability of exchange rate impacts between the U. S. dollar and the Euro. Under the terms of the cross-currency swap agreement, the Company notionally exchanged $
On February 1, 2023, the Company entered into another cross-currency swap agreement to hedge the variability of exchange rate impacts between the U.S. dollar and the Euro. Under the terms of the cross-currency swap agreement, the Company notionally exchanged $
On February 1, 2024, the Company entered into another cross-currency swap agreement to hedge the variability of exchange rate impacts between the U.S. dollar and the Euro. Under the terms of the cross-currency swap agreement, the Company notionally exchanged $
The cross-currency swaps were recorded as a derivative liability within “Accrued expenses and other current liabilities” as of September 30, 2024 and a “Derivative asset” as of September 30, 2023 in the Consolidated Balance Sheets.
Interest earned on the cross-currency swap is recorded within “Interest income, net” in the Consolidated Statements of Operations. For the fiscal years ended September 30, 2024 and 2023, the Company recorded interest income of $
7. Property, Plant and Equipment
Property, plant and equipment were as follows as of September 30, 2024 and 2023 (in thousands):
| September 30, | ||||||||
| 2024 | 2023 | |||||||
| Buildings, land, and land use right | $ | $ | ||||||
| Computer equipment and software | ||||||||
| Machinery and equipment | ||||||||
| Furniture and fixtures | ||||||||
| Leasehold improvements | ||||||||
| Capital projects in process | ||||||||
| Right-of-use asset | ||||||||
| Vehicles | ||||||||
| Property, plant and equipment, gross | ||||||||
| Less: accumulated depreciation and amortization | ( | ) | ( | ) | ||||
| Property, plant and equipment, net | $ | $ | ||||||
Depreciation expense, which includes amortization expense on finance leases, was $
As of September 30, 2024 and 2023, the Company had cumulative capitalized direct costs of $
8. Goodwill and Intangible Assets
The Company conducts an impairment assessment annually on April 1, or more frequently if impairment indicators are present. Changes to the Company’s operating segments effective October 1, 2023 resulted in a change to the Company’s reporting units, which are aligned to the Company’s operating and reportable segments (as further described in Note 18, Segment and Geographic Information below). As a result of this segment realignment, the Company allocated goodwill to the reporting units existing under the new organizational structure on a relative fair value basis as of October 1, 2023. The Company estimated the fair values of the affected businesses based upon the present value of their anticipated future cash flows. The Company's determination of fair value involved judgment and the use of significant estimates and assumptions.
The Company tested its reporting units for potential impairment immediately before and after the segment realignment and concluded that the estimated fair value of each reporting unit exceeded its respective carrying value. As of October 1, 2023, the fair value of the B Medical Systems reporting unit exceeded its carrying value by approximately 5 percent.
During the second quarter of fiscal year 2024, as part of the Company’s routine long-term planning process, the Company assessed several events and circumstances that could affect the significant inputs used to determine the fair value of its reporting units, including updates to forecasted cash flows, the impact of the Company’s planned transformation initiatives and the overall change in the economic climate since its last impairment assessment in October 2023. The Company concluded it was more likely than not the fair value of the Company’s B Medical Systems segment was less than its carrying amount resulting from the reduction in the Company’s anticipated revenue growth rates for the current and subsequent years as compared to prior projections. As a result, the Company completed a quantitative goodwill impairment test for its reporting units in accordance with ASC 350, Intangibles – Goodwill as of March 31, 2024.
For the quantitative goodwill impairment analyses performed, the Company compared the estimated fair values of each of its reporting units to their respective carrying amounts. The estimated fair values of each of the reporting units were derived using the income approach, specifically the DCF method. The discounted cash flow models used in the DCF Method analysis reflected the Company’s assumptions regarding revenue growth rates, forecast gross profit margins, operating expenses, capital expenditures, discount rates, terminal period growth rates, economic and market trends, and other expectations about the anticipated operating results of its reporting units. As part of the goodwill impairment test, the Company also considered its market capitalization and guideline public companies in assessing the reasonableness of the combined fair values estimated for its reporting units. Goodwill impairment is measured as the excess of a reporting unit's carrying amount over its estimated fair value, not to exceed the carrying amount of goodwill for that reporting unit.
The results of the Company’s quantitative goodwill impairment analyses as of March 31, 2024 indicated an impairment of goodwill within its B Medical Systems reporting unit resulting in a non-cash impairment charge of $
In the event the financial performance of any of the reporting units does not meet management’s expectations in the future, the Company experiences a prolonged macroeconomic downturn, or there are other negative revisions to key assumptions used in the DCF method used to value the reporting units, the Company may be required to perform additional impairment analyses with respect to such reporting units and could be required to recognize additional impairment charges.
The following table sets forth the changes in the carrying amount of goodwill by reportable segment that is consistent with the Company's operating segments effective October 1, 2023 (in thousands):
| Life Sciences Products | Life Sciences Services | Sample Management Solutions | Multiomics | B Medical Systems | Total | |||||||||||||||||||
| Balance - September 30, 2022 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Acquisitions | ||||||||||||||||||||||||
| Currency translation adjustments | ||||||||||||||||||||||||
| Balance - September 30, 2023 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Segment recast (1) | ( | ) | ( | ) | ||||||||||||||||||||
| Impairment | ( | ) | ( | ) | ||||||||||||||||||||
| Currency translation adjustments | ||||||||||||||||||||||||
| Balance - September 30, 2024 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Accumulated goodwill impairments, September 30, 2024 | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) |
| (1) | Changes to the Company’s operating segments effective October 1, 2023 resulted in a change to the Company’s reporting units. As a result of this segment realignment, the Company allocated goodwill to the reporting units existing under the new organizational structure on a relative fair value basis as of October 1, 2023. |
The components of the Company’s identifiable intangible assets as of September 30, 2024 and 2023 are as follows (in thousands):
| September 30, 2024 | September 30, 2023 | |||||||||||||||||||||||
| Accumulated | Net Book | Accumulated | Net Book | |||||||||||||||||||||
| Cost | Amortization | Value | Cost | Amortization | Value | |||||||||||||||||||
| Patents | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
| Completed technology | ||||||||||||||||||||||||
| Trademarks and trade names | ||||||||||||||||||||||||
| Non-competition agreements | ||||||||||||||||||||||||
| Customer relationships | ||||||||||||||||||||||||
| Other intangibles | ||||||||||||||||||||||||
| Total | $ | $ | $ | $ | $ | $ | ||||||||||||||||||
For further details regarding the goodwill and intangible assets obtained from the B Medical and Ziath acquisitions, please refer to Note 4, Business Combinations.
During the second quarter of fiscal year 2024, the Company discontinued its sample sourcing product offering (a product line within the Sample Management Solutions segment). As a result, the Company recorded a $
Amortization expense for intangible assets was $
Estimated future amortization expense for the intangible assets as of September 30, 2024 is as follows (in thousands):
| 2025 | $ | |||
| 2026 | ||||
| 2027 | ||||
| 2028 | ||||
| 2029 | ||||
| Thereafter | ||||
| Total | $ |
9. Restructuring
2024 Restructuring Plan
In the second quarter of fiscal year 2024, the Company launched initiatives designed to optimize resources for future growth and improve efficiency across its organization. The focus of the initiatives is to improve the Company’s profitability, which includes facilities consolidation, portfolio optimization, and organization structure simplification. The Company expects to complete the activities included in these initiatives by the end of fiscal year 2026. As of the date of issuance of the financial statements for the fiscal year ended September 30, 2024, the Company has not identified restructuring actions related to these initiatives that will result in additional material charges. The Company expects to identify additional actions as it further refines its plan, and the related initiatives in future periods will be recorded when specified criteria are met, including but not limited to, communication of benefit arrangements or when the costs have been incurred.
The majority of the restructuring expenses associated with the initiatives described above for the fiscal year ended September 30, 2024 are severance and related costs, operating lease related ROU asset abandonment, and fixed assets and other asset write-offs. Of the total restructuring expenses in the fiscal year ended September 30, 2024, $
2023 Cost Savings Plan
In the second and third quarters of fiscal year 2023, the Company announced cost savings plans designed to position the Company to meet the needs of its customers and accelerate growth of the business.
The majority of the restructuring expenses for fiscal years 2023 and 2022 are related to severance and related costs. The cost savings plans were completed and costs from the actions were fully realized by the end of the first quarter of fiscal year 2024.
The following table presents restructuring charges recognized for the fiscal years ended September 30, 2024 and 2023 (in thousands):
| Year Ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| Severance and related costs | $ | $ | ||||||
| Property, plant and equipment and other asset write-offs | ||||||||
| ROU asset abandonment | ||||||||
| Other | ||||||||
| Total restructuring charges | $ | $ | ||||||
The following table sets forth the activity in the severance and related costs accruals for the fiscal years ended September 30, 2024 and 2023 (in thousands):
| Year Ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| Balance at beginning of period | $ | $ | ||||||
| Provisions and adjustments | ||||||||
| Payments | ( | ) | ( | ) | ||||
| Balance at end of period | $ | $ | ||||||
10. Leases
The Company has operating and finance leases for real estate and other assets in North America, Europe, and Asia. Non-real estate leases are primarily related to vehicles and office equipment. Lease expiration dates range between 2024 and 2043.
The components of lease expense for fiscal years 2024 and 2023 are as follows (in thousands):
| Year Ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| Operating lease costs | $ | $ | ||||||
| Finance lease costs: | ||||||||
| Amortization of assets | ||||||||
| Interest on lease liabilities | ||||||||
| Total finance lease costs | ||||||||
| Total operating and finance lease costs | ||||||||
| Variable lease costs | ||||||||
| Short-term lease costs | ||||||||
| Total lease costs | $ | $ | ||||||
Supplemental balance sheet information related to leases is as follows (in thousands, except lease term and discount rate):
| September 30, 2024 | September 30, 2023 | |||||||
| Operating Leases: | ||||||||
| Operating lease right-of-use assets | $ | $ | ||||||
|
| $ | $ | ||||||
| Long-term operating lease liabilities | ||||||||
| Total operating lease liabilities | $ | $ | ||||||
| Finance Leases: | ||||||||
| Property, plant and equipment, at cost | $ | $ | ||||||
| Accumulated amortization | ( | ) | ( | ) | ||||
| Property, plant and equipment, net | $ | $ | ||||||
|
| $ | $ | ||||||
|
| ||||||||
| Total finance lease liabilities | $ | $ | ||||||
| Weighted average remaining lease term (in years): | ||||||||
| Operating leases | ||||||||
| Finance leases | ||||||||
| Weighted average discount rate: | ||||||||
| Operating leases | % | % | ||||||
| Finance leases | % | % | ||||||
Supplemental cash flow information related to leases is as follows (in thousands):
| Year Ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| Cash paid for amounts included in measurement of liabilities: | ||||||||
| Operating cash flows - operating leases | $ | $ | ||||||
| Operating cash flows - finance leases | $ | $ | ||||||
| Financing cash flows - finance leases | $ | $ | ||||||
| ROU assets obtained in exchange for lease liabilities: | ||||||||
| Operating leases | $ | $ | ||||||
| Finance leases | $ | $ | ||||||
Future lease payments for operating leases as of September 30, 2024 are as follows for the subsequent five fiscal years and thereafter (in thousands):
| Finance Leases | Operating Leases | |||||||
| 2025 | $ | $ | ||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| 2029 | ||||||||
| Thereafter | ||||||||
| Total future lease payments | ||||||||
| Less imputed interest | ( | ) | ( | ) | ||||
| Total lease liability balance | $ | $ | ||||||
11. Supplementary Balance Sheet Information
The following is a summary of accounts receivable at September 30, 2024 and 2023 (in thousands):
| September 30, | ||||||||
| 2024 | 2023 | |||||||
| Accounts receivable | $ | $ | ||||||
| Less allowance for expected credit losses | ( | ) | ( | ) | ||||
| Accounts receivable, net | $ | $ | ||||||
The allowance for expected credit losses for the fiscal years ended September 30, 2024, 2023 and 2022 is as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Balance at beginning of period | $ | $ | $ | |||||||||
| Provisions | ||||||||||||
| Payments received | ( | ) | ( | ) | ( | ) | ||||||
| Write-offs and adjustments | ( | ) | ( | ) | ( | ) | ||||||
| Balance at end of period | $ | $ | $ | |||||||||
The following is a summary of inventories at September 30, 2024 and 2023 (in thousands):
| September 30, | ||||||||
| 2024 | 2023 | |||||||
| Raw materials and purchased parts | $ | $ | ||||||
| Work-in-process | ||||||||
| Finished goods | ||||||||
| Total inventories | $ | $ | ||||||
The activity for excess and obsolete inventory reserves is as follows for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Balance at beginning of period | $ | $ | $ | |||||||||
| Provisions | ||||||||||||
| Inventory disposals and adjustments | ( | ) | ( | ) | ( | ) | ||||||
| Balance at end of period | $ | $ | $ | |||||||||
The activity for valuation allowance for deferred tax assets is as follows for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Balance at beginning of period | $ | $ | $ | |||||||||
| Charge to income tax provision (benefit) | ||||||||||||
| Charged to other accounts | ( | ) | ||||||||||
| Balance at end of period | $ | $ | $ | |||||||||
The following is a summary of product warranty and retrofit activity on a gross basis for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Balance at beginning of period | $ | $ | $ | |||||||||
| Adjustments for acquisitions and divestitures | ||||||||||||
| Accruals for warranties during the year | ||||||||||||
| Costs incurred during the year | ( | ) | ( | ) | ( | ) | ||||||
| Balance at end of period | $ | $ | $ | |||||||||
12. Stockholders’ Equity
Share Repurchases
On November 4, 2022, the Company's Board of Directors approved an authorization to repurchase up to $
In April 2023, other arrangements commenced under the 2022 Repurchase Authorization with the intent of repurchasing the remaining $
Effective January 1, 2023, all corporate share repurchases are subject to a one percent excise tax on the value of the repurchase, net of share issuances, subject to certain exclusions. The excise tax was part of The Inflation Reduction Act passed by the U.S. government in 2022. The Company accrued $
Preferred Stock
Total number of shares of preferred stock authorized for issuance was
Accumulated Other Comprehensive Income (Loss)
The following is a summary of the components of accumulated other comprehensive income (loss), net of tax, at September 30, 2024, 2023 and 2022 (in thousands):
| Unrealized | ||||||||||||||||||||
| Gains (Losses) | ||||||||||||||||||||
| on Available- | Gains (Losses) | |||||||||||||||||||
| Currency | for-Sale | on Derivative | Pension | |||||||||||||||||
| Translation | Securities | asset | Liability | |||||||||||||||||
| Adjustments | Net of tax | Net of tax | Adjustments | Total | ||||||||||||||||
| Balance at September 30, 2021 | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ( | ) | ||||||||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | ( | ) | ( | ) | ||||||||||||||||
| Balance at September 30, 2022 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ||||||||||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | ||||||||||||||||||||
| Balance at September 30, 2023 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ) | ( | ) | ||||||||||||||||
| Amounts reclassified from accumulated other comprehensive income (loss) | ||||||||||||||||||||
| Balance at September 30, 2024 | $ | ( | ) | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | |||||||
Unrealized gains (losses) on available-for-sale marketable securities are reclassified from “Accumulated other comprehensive income (loss)” into results of operations at the time of the securities’ sale, as described in Note 5, Marketable Securities. Amounts reclassified from accumulated other comprehensive income (loss) related to pension liability adjustments represent amortization of actuarial gains and losses.
13. Revenue from Contracts with Customers
Disaggregated Revenue
The Company disaggregates revenue from contracts with customers in a manner that depicts how the nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factors. The following is revenue by significant business line for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| 2024 | 2023 | 2022 | ||||||||||
| Significant Business Line | ||||||||||||
| Multiomics | $ | $ | $ | |||||||||
| Core Products(1) | ||||||||||||
| Sample Repository Services | ||||||||||||
| B Medical Systems | ||||||||||||
| Total revenue | $ | $ | $ | |||||||||
| (1) Core Products are Automated Stores, Cryogenic Systems, Automated Sample Tube, Consumables and Instruments and Controlled Rate Thawing Devices. |
Contract Balances
Accounts Receivable, Net. Accounts receivable represent rights to consideration in exchange for products or services that have been transferred by the Company, when payment is unconditional and only the passage of time is required before payment is due. Accounts receivable do not bear interest and are recorded at the invoiced amount. The Company maintains an allowance for expected credit losses representing its best estimate of probable credit losses related to its existing accounts receivable and their net realizable value. The Company determines the allowance for expected credit losses based on a number of factors, including an evaluation of customer credit worthiness, the age of the outstanding receivables, economic trends, historical experience, and other information through the payment periods. Accounts receivable, net were $
Contract Assets. Contract assets represent rights to consideration in exchange for products or services that have been transferred by the Company, when payment is conditional on something other than the passage of time. These amounts typically relate to contracts where the right to invoice the customer is not present until completion of the contract or the achievement of specified milestones and the value of the products or services transferred exceed this constraint. Contract assets are classified as current as they are expected to convert to cash within one year. Contract asset balances which are included within “Prepaid expenses and other current assets” on the Company’s Consolidated Balance Sheet, were $
Contract Liabilities. Contract liabilities represent the Company’s obligation to transfer products or services to a customer for which consideration has been received, or for which an amount of consideration is due from the customer. Contract assets and liabilities are reported on a net basis at the contract level, depending on the contracts position at the end of each reporting period. Contract liabilities are included within “Deferred revenue” on the Company’s Consolidated Balance Sheet. Contract liabilities were $
Remaining Performance Obligations. Remaining performance obligations represent the transaction price of unsatisfied or partially satisfied performance obligations within contracts with an original expected contract term that is greater than one year and for which fulfillment of the contract has started as of the end of the reporting period. The aggregate amount of transaction consideration allocated to remaining performance obligations as of September 30, 2024 was $
| As of September 30, 2024 | ||||||||||||
| Less than Year | Greater than Year | Total | ||||||||||
| Remaining performance obligations | $ | $ | $ | |||||||||
Cost to Obtain and Fulfill a Contract
The Company capitalizes sales commissions when incurred if they are (i) incremental costs of obtaining a contract, (ii) expected to be recovered and (iii) have an expected amortization period that is greater than one year. These amounts primarily relate to sales commissions and are being amortized over a -month period, which represents the average period of contract performance. The capitalized sales commissions were $
The Company accounts for shipping and handling activities as fulfillment activities and recognize the associated expense when control of the product has transferred to the customer.
14. Stock Based Compensation
In accordance with the 2020 Equity Incentive Plan, the Company may issue eligible employees options to purchase shares of the Company’s common stock, restricted stock units and other equity incentives, which vest upon the satisfaction of a performance condition and/or a service condition. In addition, the Company issues common stock to participating employees pursuant to an employee stock purchase plan, and may issue common stock awards and deferred restricted stock units to members of its Board of Directors in accordance with its Board of Directors compensation program.
2020 Equity Incentive Plan
In accordance with the 2020 Equity Incentive Plan (the “2020 Plan”), the Company may grant employees (i) restricted stock and other stock-based awards, (ii) nonqualified stock options, and (iii) options intended to qualify as incentive stock options under Section 422 of the Internal Revenue Code. All employees of the Company or any affiliate of the Company, independent directors, consultants and advisors are eligible to participate in the 2020 Plan. The 2020 Plan provides for the issuance of an aggregate of
The following table reflects stock-based compensation expense recorded during the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Restricted stock units | $ | $ | $ | |||||||||
| Employee stock purchase plan | ||||||||||||
| Total stock-based compensation expense for continuing and discontinued operations | $ | $ | $ | |||||||||
| Income tax benefit | ( | ) | ( | ) | ( | ) | ||||||
| Total compensation expense included in the statement of operations | $ | $ | $ | |||||||||
Restricted Stock Unit Activity
The following table summarizes restricted stock unit activity for the fiscal year ended September 30, 2024:
| Weighted | ||||||||
| Average | ||||||||
| Shares | Grant Date | |||||||
| (in thousands) | Fair Value | |||||||
| Outstanding as of September 30, 2023 | $ | |||||||
| Granted | $ | |||||||
| Vested | ( | ) | $ | |||||
| Forfeited | ( | ) | $ | |||||
| Outstanding as of September 30, 2024 | $ | |||||||
The fair value of restricted stock units vested during fiscal years 2024, 2023 and 2022 was $
The following table reflects restricted stock units and stock awards granted during fiscal years ended September 30, 2024, 2023 and 2022:
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Time-based restricted stock units | ||||||||||||
| Common stock awards | ||||||||||||
| Performance-based restricted stock units | ||||||||||||
| Total units | ||||||||||||
Time-Based Restricted Stock Unit Grants
Restricted stock units granted with a required service period typically have -year vesting schedules in which -third of awards vest at each annual anniversary of grant date, subject to the award holders meeting service requirements.
Certain members of the Board of Directors have elected to defer receiving their annual stock awards and related quarterly dividends, if any, until they attain a certain age or cease to provide services as a member of the Company’s Board of Directors. Annual deferred stock awards granted during fiscal years 2024, 2023, and 2022 vested upon issuance.
Performance-Based Restricted Stock Unit Grants
Performance-based restricted stock units are earned based on the achievement of performance criteria established by the Human Resources and Compensation Committee and approved by the Board of Directors. The criteria for performance-based awards are weighted and have threshold, target and maximum performance goals. Performance-based restricted stock units may also have a required service period following the achievement of all or a portion of the performance goals.
Performance-based restricted stock unit awards granted in fiscal year 2024, 2023 and 2022 allow participants to earn
In October 2023, the Company’s Board of Directors approved an amendment to the performance goals associated with the previously issued performance-based restricted stock units for all impacted employees, excluding members of the Company’s executive team. The performance goals, as amended, are more reflective of the current macroeconomic environment and consideration toward employee retention in the competitive life sciences industry. Before the amendment, the original performance goals were not expected to be satisfied. Subsequent to the amendment, vesting became probable based on the forecasted achievement of the amended performance goals. The amendment of these restricted stock units is treated as a modification with the total potential maximum compensation cost of $
Awards Granted to the Board of Directors
The stock-based compensation granted to members of the Company’s Board of Directors includes common stock awards, restricted stock unit awards and deferred common stock and restricted stock unit awards.
Employee Stock Purchase Plan
The Company maintains an employee stock purchase plan that allows its employees to purchase shares of common stock at a price equal to
Valuation Assumptions for an Employee Stock Purchase Plan
The fair value of shares issued under the employee stock purchase plan is estimated on the commencement date of each offering period using the Black-Scholes option-pricing model with the following weighted average assumptions for the fiscal years ended September 30, 2024, 2023 and 2022:
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Risk-free interest rate | % | % | % | |||||||||
| Volatility | % | % | % | |||||||||
| Expected life | ||||||||||||
| Dividend yield | % | % | % | |||||||||
The risk-free rate is based on the U.S. Treasury yield curve for notes with terms approximating the expected life of the shares granted. The expected stock price volatility is determined based on the Company’s historic stock prices over a period commensurate with the expected life of the shares granted. The expected life represents the weighted average period over which the shares are expected to be purchased. Dividend yields are projected based on the Company’s history of dividend declarations and management’s intention for future dividend declarations.
15. Fair Value Measurements
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following tables summarize assets and liabilities measured and recorded at fair value on a recurring basis in the accompanying Consolidated Balance Sheets as of September 30, 2024 and 2023 (in thousands):
| As of September 30, 2024 | ||||||||||||||||
| Description | Total Fair Value | Level 1 | Level 2 | Level 3 | ||||||||||||
| Assets: | ||||||||||||||||
| Cash equivalents | $ | $ | $ | $ | ||||||||||||
| Available-for-sale securities | ||||||||||||||||
| Convertible debt securities | ||||||||||||||||
| Foreign exchange contracts | ||||||||||||||||
| Total assets | $ | $ | $ | $ | ||||||||||||
| Liabilities: | ||||||||||||||||
| Net investment hedge | ||||||||||||||||
| Foreign exchange contracts | ||||||||||||||||
| Total liabilities | $ | $ | $ | $ | ||||||||||||
| As of September 30, 2023 | ||||||||||||||||
| Description | Total Fair Value | Level 1 | Level 2 | Level 3 | ||||||||||||
| Assets: | ||||||||||||||||
| Cash equivalents | $ | $ | $ | $ | ||||||||||||
| Available-for-sale securities | ||||||||||||||||
| Foreign exchange contracts | ||||||||||||||||
| Net investment hedge | ||||||||||||||||
| Total assets | $ | $ | $ | $ | ||||||||||||
| Liabilities: | ||||||||||||||||
| Foreign exchange contracts | ||||||||||||||||
| Total liabilities | $ | $ | $ | $ | ||||||||||||
Cash Equivalents
Cash equivalents consist of money market funds and are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices in active markets. The Company considers all highly liquid interest-earning investments with a maturity of three months or less at the date of purchase to be cash equivalents. The fair values of these investments approximate their carrying values.
Available-For-Sale Securities
Available-for-sale securities primarily consist of highly rated corporate debt securities and U.S. government backed securities which are classified as Level 1. Investments classified as Level 2 consist of debt securities that are valued using matrix pricing and benchmarking because they are not actively traded, and bank certificates of deposit. Matrix pricing is a mathematical technique used to value securities by relying on the securities’ relationship to other benchmark quoted prices.
Convertible Debt Securities
In the third quarter of fiscal year 2024, the Company purchased $
Foreign Exchange Contracts & Net Investment Hedge
Our foreign exchange contract assets and liabilities, and our net investment hedge assets and liabilities are measured and reported at fair value using the market method valuation technique. The inputs to this technique utilize current foreign currency exchange forward market rates published by third-party leading financial news and data providers. These are observable data that represent the rates that the financial institution uses for contracts entered into at that date; however, they are not based on actual transactions, so they are classified as Level 2.
Assets and Liabilities Measured at Fair Value on a Nonrecurring Basis
In addition to assets and liabilities that are recorded at fair value on a recurring basis, impairment indicators may subject goodwill and long-lived assets to fair value measurement on a nonrecurring basis. As described in Note 8, Goodwill and Intangible Assets, as of September 30, 2024, the Company estimated the fair value of its reporting units using the DCF Method. Because the inputs to the valuation method are largely unobservable and reflect the Company’s own assumptions, goodwill and long-lived assets are classified as Level 3.
Contingent Consideration Liability
The contingent consideration liability related to the acquisition of B Medical was measured and reported at fair value using the real options method based on the unobservable inputs that were significant to the fair value and classified with Level 3 of the fair value hierarchy. The contingency was based on the acquired business’ performance through September 30, 2023. Please refer to Note 4, Business Combinations for further details. This liability was revalued from $
16. Income Taxes
The components of the income tax (benefit) expense from continuing operations for the fiscal years are as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Current income tax (benefit) expense | ||||||||||||
| Federal | $ | $ | ( | ) | $ | ( | ) | |||||
| State | ||||||||||||
| Foreign | ||||||||||||
| Total current income tax (benefit) expense | ||||||||||||
| Deferred income tax (benefit) expense: | ||||||||||||
| Federal | ( | ) | ( | ) | ( | ) | ||||||
| State | ( | ) | ( | ) | ||||||||
| Foreign | ( | ) | ( | ) | ||||||||
| Total deferred income tax (benefit) expense | ( | ) | ( | ) | ||||||||
| Income tax (benefit) expense | $ | ( | ) | $ | ( | ) | $ | |||||
The components of income (loss) from continuing operations before income taxes for the fiscal years are as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Domestic | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Foreign | ( | ) | ||||||||||
| Loss before income taxes | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
The differences between the income tax (benefit) expense on income (loss) from continuing operations and income taxes computed using the applicable U.S. statutory federal tax rates for the fiscal years ended September 30, 2024, 2023 and 2022 are as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Income tax benefit computed at federal statutory rate | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| State income taxes, net of federal benefit | ( | ) | ( | ) | ( | ) | ||||||
| Foreign income taxed at different rates | ( | ) | ( | ) | ( | ) | ||||||
| Impact of investments in subsidiaries | ( | ) | ( | ) | ||||||||
| Nontaxable gain from acquisition earn-out liability reversal | ( | ) | ||||||||||
| Change in deferred tax asset valuation allowance | ||||||||||||
| Impact of change in uncertain tax positions | ( | ) | ( | ) | ||||||||
| Global intangible low taxed income, net of foreign tax credits | ||||||||||||
| Impact of tax rate changes | ( | ) | ( | ) | ||||||||
| Compensation | ( | ) | ||||||||||
| Tax credits | ( | ) | ( | ) | ( | ) | ||||||
| Merger costs | ||||||||||||
| Other non-deductible expenses and other taxes | ||||||||||||
| Impact of effective state tax rate change | ||||||||||||
| Goodwill impairment | ||||||||||||
| Research and development expense deduction | ( | ) | ( | ) | ( | ) | ||||||
| Income tax (benefit) expense | $ | ( | ) | $ | ( | ) | $ | |||||
During the fiscal year 2024, the Company repatriated approximately $
During fiscal year 2024, the Company recorded a goodwill impairment charge related to the B Medical Systems segment. This impairment charge is not deductible for tax purposes. For tax purposes the Company has estimated that Azenta Luxembourg, the owner of B Medical Systems, would have a deductible impairment of its investment in the business, which is a significant component of the $22.6 million rate reconciliation benefit for “Impact of investments in subsidiaries” above. This tax deduction puts Luxembourg into a valuation allowance position as it does not have sufficient taxable income to utilize the loss, therefore driving a significant increase in the valuation allowance.
The Company has not provided deferred income taxes on the outside basis differences of any other foreign subsidiary and maintains its general assertion of indefinite reinvestment regarding those subsidiaries and the remaining earnings of its German subsidiary as of September 30, 2024. The remaining foreign earnings total approximately $
The significant components of the net deferred tax assets and liabilities as of September 30, 2024 and 2023 are as follows (in thousands):
| September 30, | ||||||||
| 2024 | 2023 | |||||||
| Accruals and reserves not currently deductible | $ | $ | ||||||
| Federal, state and foreign tax credits | ||||||||
| Other assets | ||||||||
| Equity compensation | ||||||||
| Net operating loss carryforwards | ||||||||
| Lease liabilities | ||||||||
| Capitalized research and development | ||||||||
| Deferred revenue | ||||||||
| Outside basis differences in subsidiaries | ||||||||
| Deferred tax assets | ||||||||
| Depreciation and intangible amortization | ( | ) | ( | ) | ||||
| Right-of-use assets | ( | ) | ( | ) | ||||
| Other liabilities | ( | ) | ( | ) | ||||
| Net unrealized gain on hedging and investments | ( | ) | ||||||
| Deferred tax liabilities | ( | ) | ( | ) | ||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred tax asset (liability) | $ | ( | ) | $ | ( | ) | ||
The deferred tax assets on the balance sheets for September 30, 2024 and 2023 also include $
ASC Topic 740 requires that all available evidence, both positive and negative, be considered in determining, based on the weight of that evidence, whether a valuation allowance is needed. The weight given to the potential effect of negative and positive evidence should be commensurate with the extent to which it can be objectively verified. The more negative evidence that exists, (a) the more positive evidence is necessary and (b) the more difficult it is to support a conclusion that a valuation allowance is not needed for some portion or the entire deferred tax asset.
The Company evaluates the realizability of its deferred tax assets and assesses the need for a valuation allowance on a quarterly basis. The Company operates in numerous countries under many legal forms and, as a result, is subject to the jurisdiction of numerous domestic and foreign tax authorities. The Company evaluates the profitability of its operations in each jurisdiction on a historic cumulative basis and on a forward-looking basis, while carefully considering carry-forward periods of tax attributes and ongoing tax planning strategies in assessing the need for the valuation allowance.
The Company has generated U.S. pre-tax losses in recent years but has been in an overall U.S. deferred tax liability position. As of September 30, 2024, the Company is in a net U.S. deferred tax asset position and considered whether the future taxable temporary differences are sufficient to offset future deductible temporary differences. As a result, an additional valuation allowance was recorded against U.S. federal and state deferred tax assets during fiscal year 2024. After evaluating all the relevant positive and negative evidence, the Company has recorded a $
As of September 30, 2024, the Company has tax-effected federal, state and foreign net operating loss carry-forwards of approximately $
The Company has performed studies to determine if there are any annual limitations on the federal net operating losses under Section 382 of the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code. As a result of these studies, the Company has determined that ownership changes have occurred primarily in connection with acquisitions when the Company has issued stock to the sellers, as well as ownership changes in the subsidiaries acquired by the Company. The benefits of the net operating losses that will expire before utilization have not been recorded as deferred tax assets in the accompanying Consolidated Balance Sheets.
The Company maintains liabilities for unrecognized tax benefits. These liabilities involve judgment and estimation, and they are monitored based on the best information available. A reconciliation of the beginning and ending amount of the consolidated liability for unrecognized income tax benefits during the fiscal years ended September 30, 2024, 2023 and 2022 is as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Balance at beginning of period | $ | $ | $ | |||||||||
| Reductions from lapses in statutes of limitation | ( | ) | ( | ) | ||||||||
| Balance at end of period | $ | $ | $ | |||||||||
All of the unrecognized tax benefits for the fiscal year ended September 30, 2024 would impact the effective tax rate if recognized. The Company recognizes interest related to unrecognized benefits as a component of the income tax (benefit) expense which was not material for the fiscal years ended September 30, 2024, 2023 and 2022.
The Company is subject to U.S. federal, state, local and foreign income taxes in various jurisdictions. The amount of income taxes paid is subject to the Company’s interpretation of applicable tax laws in the jurisdictions in which it files.
In the normal course of business, the Company is subject to income tax audits in various global jurisdictions in which it operates. The years subject to examination vary for the United States and international jurisdictions, with the earliest tax year being 2019. Based on the outcome of these examinations or the expiration of statutes of limitations for specific jurisdictions, it is reasonably possible that the related unrecognized tax benefits could change from those recorded in the Company’s Consolidated Balance Sheets. The Company currently anticipates that it is reasonably possible that the unrecognized tax benefits and accrued interest on those benefits will be reduced by $
17. Net Income (Loss) per Share
The following table shows the computation of basic and diluted loss (income) per share for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands, except per share data):
| Year Ended September 30, |
||||||||||||
| 2024 |
2023 |
2022 |
||||||||||
| Loss from continuing operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| (Loss) income from discontinued operations, net of tax |
( |
) | ||||||||||
| Net (loss) income |
( |
) | ( |
) | ||||||||
| Weighted average common shares outstanding used in computing basic (loss) income per share |
||||||||||||
| Weighted average common shares outstanding used in computing diluted (loss) income per share |
||||||||||||
| Basic net (loss) income per share: |
||||||||||||
| Loss from continuing operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| (Loss) income from discontinued operations, net of tax |
( |
) | ||||||||||
| Basic net (loss) income per share |
$ | ( |
) | $ | ( |
) | $ | |||||
| Diluted net (loss) income per share: |
||||||||||||
| Loss from continuing operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| (Loss) income from discontinued operations, net of tax |
( |
) | ||||||||||
| Diluted net (loss) income per share |
$ | ( |
) | $ | ( |
) | $ | |||||
18. Segment and Geographic Information
Operating segments are defined as components of an enterprise that engage in business activities for which discrete financial information is available and regularly reviewed by the chief operating decision maker (“CODM”) in deciding how to allocate resources and to assess performance. The Company’s Chief Executive Officer is the Company’s CODM.
Effective October 1, 2023, the Company realigned its organizational structure to principal business segments to enhance its commercial strategy for accelerating growth and to enable additional profitability initiatives. These segments align with changes in how the Company’s CODM manages the business, allocates resources, and assesses performance. The Company’s operating and reportable segments consist of the following:
| ● | Sample Management Solutions. The SMS business resources operate as a single business unit offering end-to-end sample management products and services, including: Sample Repository Services and Core Products (Automated Stores, Cryogenic Systems, Automated Sample Tube, Consumables and Instruments and Controlled Rate Thawing Devices). |
| ● | Multiomics. The Multiomics business resources operate as a single business unit offering genomic and other sample analysis services, including gene sequencing, synthesis editing and related services. |
| ● | B Medical Systems. The B Medical Systems business resources operate as a single business unit focused on the manufacturing and distribution of temperature-controlled storage and transportation solutions in international markets to governments, health institutions, and non-government organizations. |
The segment realignment had no impact on the Company’s consolidated financial position, results of operations, or cash flows. All segment information is reflective of this new structure, and prior period information has been recast to conform to our current period presentation.
Management considers adjusted operating income (loss), which excludes charges related to amortization of intangible assets, purchase accounting impact on inventory, transformation and rebranding costs, restructuring charges, goodwill and intangible asset impairment, fair value adjustments to contingent consideration, merger and acquisition costs and costs related to share repurchase, governance-related matters, and other unallocated corporate expenses, as the primary performance metric when evaluating each segment’s operations.
The following is the summary of the financial information for the Company’s reportable segments for the fiscal years ended September 30, 2024, 2023 and 2022 (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Revenue: | ||||||||||||
| Sample Management Solutions | $ | $ | $ | |||||||||
| Multiomics | ||||||||||||
| B Medical Systems | ||||||||||||
| Total revenue | $ | $ | $ | |||||||||
| Adjusted operating income (loss): | ||||||||||||
| Sample Management Solutions | $ | $ | ( | ) | $ | |||||||
| Multiomics | ( | ) | ( | ) | ||||||||
| B Medical Systems | ( | ) | ||||||||||
| Segment adjusted operating income (loss) | ( | ) | ( | ) | ||||||||
| Amortization of completed technology | ||||||||||||
| Purchase accounting impact on inventory | ||||||||||||
| Amortization of intangible assets other than completed technology | ||||||||||||
| Transformation(1) and rebranding costs | ( | ) | ||||||||||
| Restructuring charges | ||||||||||||
| Impairment of goodwill and intangible assets | ||||||||||||
| Contingent consideration - fair value adjustments | ( | ) | ||||||||||
| Merger and acquisition costs and costs related to share repurchase(2) | ||||||||||||
| Other unallocated corporate expenses | ||||||||||||
| Total operating loss | ( | ) | ( | ) | ( | ) | ||||||
| Interest income, net | ||||||||||||
| Other income (expense), net | ( | ) | ( | ) | ||||||||
| Loss before income taxes | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| (1) | Transformation costs represent non-recurring expenses for strategic projects with anticipated long-term benefits to the Company focused on cost reduction and productivity improvement that do not meet the definition of restructuring charges. These costs are directed at simplifying, standardizing, streamlining, and optimizing the Company’s operations, processes and systems to permanently alter the Company’s operations for the long term. For a project to be considered transformational, successful completion of the project must be expected to bring long-term material benefits to the organization and involve significant changes to process and/or underlying technology. Transformation costs in the period result from actions taken as part of the Company’s 2024 cost reduction plan, and primarily relate to one time asset write downs associated with changes in technology, one time inventory write downs relating to restructuring actions taken in the period, and third-party consulting costs associated with process and systems re-design. |
| (2) | Includes expenses related to governance-related matters. |
The Company has corrected the segment adjusted operating income (loss) for the years ended September 30, 2023 and 2022 as certain corporate expenses that are not part of the Company’s CODM’s review of operating segment performance were improperly included in the previously disclosed segment operating income (loss) performance measure. As a result, the presentation of amounts previously disclosed for segment operating income (loss) were updated to reflect segment adjusted operating income (loss) after having been adjusted to remove these corporate expenses. These adjustments reduced total segment adjusted operating loss for fiscal year 2023 by $
The following is the summary of the asset information for the Company's reportable segments as of September 30, 2024 and 2023 (in thousands):
| Assets: | September 30, 2024 | September 30, 2023 | ||||||
| Sample Management Solutions | $ | $ | ||||||
| Multiomics | ||||||||
| B Medical Systems | ||||||||
| Total assets | $ | $ | ||||||
The following is a reconciliation of the segment assets to the corresponding amounts presented in the Consolidated Balance Sheets as of September 30, 2024 and 2023 (in thousands):
| September 30, | September 30, | |||||||
| 2024 | 2023 | |||||||
| Segment assets | $ | $ | ||||||
| Cash and cash equivalents, restricted cash, and marketable securities | ||||||||
| Deferred tax assets | ||||||||
| Other assets | ||||||||
| Total assets | $ | $ | ||||||
Revenue from external customers is attributed to geographic areas based on locations in which the product is shipped. Net revenue by geographic area for the fiscal years ended September 30, 2024, 2023 and 2022 are as follows (in thousands):
| Year Ended September 30, | ||||||||||||
| 2024 | 2023 | 2022 | ||||||||||
| Geographic Location: | ||||||||||||
| United States | $ | $ | $ | |||||||||
| Africa | ||||||||||||
| China | ||||||||||||
| United Kingdom | ||||||||||||
| Rest of Europe | ||||||||||||
| Asia Pacific/ Other | ||||||||||||
| Total revenue | $ | $ | $ | |||||||||
Net property, plant and equipment by geographic area as of September 30, 2024 and 2023 is as follows (in thousands):
| September 30, | ||||||||
| 2024 | 2023 | |||||||
| United States | $ | $ | ||||||
| China | ||||||||
| Europe | ||||||||
| Asia Pacific/ Other | ||||||||
| Total property, plant, and equipment, net | $ | $ | ||||||
Significant Customers
The Company had customer that accounted for
19. Commitments and Contingencies
Contingencies
The Company is subject to various legal proceedings, both asserted and unasserted, that arise in the ordinary course of business. The Company cannot predict the ultimate outcome of such legal proceedings or, in certain instances, provide reasonable ranges of potential losses.
The Company may also have certain indemnification obligations pursuant to claims made under the definitive agreement it entered into with Edwards Vacuum LLC (a member of the Atlas Copco Group) in connection with the Company’s sale of its semiconductor cryogenics business in the fourth quarter of fiscal year 2018. In the third quarter of fiscal year 2020, Edwards asserted claims for indemnification under the definitive agreement relating to alleged breaches of representations and warranties relating to customer warranty claims and inventory (the “2020 Claim”). In addition, in January 2023, Edwards filed a lawsuit against the Company in the Supreme Court of the State of New York in the County of New York seeking indemnification from the Company under such definitive agreement for $
In April 2023, the Company responded to and filed a counterclaim against Edwards for the 2023 Claim alleging breach of the definitive agreements by Edwards and seeking a declaratory judgment. During the third quarter of fiscal year 2023, the Company and Edwards entered into a settlement agreement related to the 2023 Claim to avoid the costs and uncertainties of potential litigation. Under the settlement agreement, the Company paid Edwards $
The Company accrued a liability of $
The Company cannot determine the probability of any losses or outcome of the 2020 Claim including the amount of any indemnifiable losses, if any, resulting from these claims. However, the Company does not believe that this claim will have a material adverse effect on its consolidated financial position or results of operations. If the resolution of the 2020 Claim results in indemnifiable losses in excess of the applicable indemnification deductibles established under the definitive agreement, Edwards would be required to seek recovery under the representation and warranty insurance Edwards obtained in connection with the closing of the sale of the semiconductor cryogenics business. Management believes that any indemnifiable losses in excess of the applicable deductibles established in the definitive agreement would be covered by such insurance. For indemnifiable claims other than those arising from breaches of representations and warranties and for indemnifiable claims arising from breaches of representations and warranties exceeding the maximum coverage of the representations and warranties insurance policy, Edwards could seek recovery of such indemnifiable losses, if any, directly from the Company. In the event of unexpected subsequent developments and given the inherent unpredictability of these matters, there can be no assurance that the Company’s assessment of any claim will reflect the ultimate outcome, and an adverse outcome in certain matters could, from time to time, have a material adverse effect on the Company’s consolidated financial position or results of operations in particular quarterly or annual periods.
Tariff Matter
With the assistance of a third-party consultant, during the first quarter of fiscal year 2021, the Company initiated a review of the value of transactions it used for intercompany imports into the U. S. from its GENEWIZ business. As a result of this review and a new interpretation surrounding the valuation method used to calculate the estimated transaction value, the Company revised its estimate of the tariffs owed and paid $
Purchase Commitments
At September 30, 2024, the Company had non-cancellable commitments of $
20. Subsequent Events
On November 12, 2024, the Company announced it is pursuing a sale of its B Medical Systems segment. The Company has concluded that, as of November 12, 2024, B Medical Systems is a discontinued operation and will be classified as held for sale in future filings. The Company is unable to provide an estimate of the financial impact of a sale transaction.
On November 12, 2024, the Company appointed Lawrence Lin as its Executive Vice President and Chief Financial Officer, effective after the filing of this Annual Report on Form 10-K, to succeed the Company’s current Executive Vice President and Chief Financial Officer, Herman Cueto, who is departing from his role at the Company. After the filing of this Annual Report on Form 10-K, Mr. Cueto will remain with the Company as an employee advisor through December 1, 2024, and thereafter as a consultant to facilitate the transition.
21. Revision of Previously Issued Unaudited Quarterly Information
The Condensed Consolidated Statements of Cash Flows for the interim periods ended March 31, 2023, June 30, 2023, December 31, 2023, March 31, 2024, and June 30, 2024 have been revised to correct for prior period errors as discussed in Note 2, Summary of Significant Accounting Policies. The effect on the Condensed Consolidated Statement of Cash Flows for each affected period is as follows (in thousands):
| Nine months ended June 30, 2023 | ||||||||||||
| (unaudited) | As Reported | Adjustments | As Revised | |||||||||
| Cash flows from operating activities | ||||||||||||
| Accrued compensation and tax withholdings | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Other current assets and liabilities | ( | ) | ( | ) | ||||||||
| Net cash used in operating activities | $ | ( | ) | $ | $ | ( | ) | |||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of common stock | $ | $ | $ | |||||||||
| Net cash used in financing activities | $ | ( | ) | $ | $ | ( | ) | |||||
| Effects of exchange rate changes on cash and cash equivalents | $ | $ | ( | ) | $ | |||||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
| Six months ended March 31, 2023 | ||||||||||||
| (unaudited) | As Reported | Adjustments | As Revised | |||||||||
| Cash flows from operating activities | ||||||||||||
| Accrued compensation and tax withholdings | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Net cash used in operating activities | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of common stock | $ | $ | $ | |||||||||
| Net cash used in financing activities | $ | ( | ) | $ | $ | ( | ) | |||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
| Nine months ended June 30, 2024 | ||||||||||||
| (unaudited) | As Reported | Adjustments | As Revised | |||||||||
| Cash flows from operating activities | ||||||||||||
| Inventories | $ | $ | $ | |||||||||
| Other assets and liabilities | ( | ) | ||||||||||
| Net cash provided by operating activities | $ | $ | ( | ) | $ | |||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property, plant and equipment | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Net cash provided by investing activities | $ | $ | ( | ) | $ | |||||||
| Effects of exchange rate changes on cash and cash equivalents | $ | $ | $ | |||||||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
| Six months ended March 31, 2024 | ||||||||||||
| (unaudited) | As Reported | Adjustments | As Revised | |||||||||
| Cash flows from operating activities | ||||||||||||
| Non-cash write-offs of assets | $ | $ | $ | |||||||||
| Inventories | ||||||||||||
| Accrued compensation and tax withholdings | ( | ) | ( | ) | ( | ) | ||||||
| Other assets and liabilities | ( | ) | ||||||||||
| Net cash provided by operating activities | $ | $ | ( | ) | $ | |||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property, plant and equipment | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Net cash used in investing activities | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Cash flows from financing activities | ||||||||||||
| Proceeds from issuance of common stock | $ | $ | $ | |||||||||
| Net cash used in financing activities | $ | ( | ) | $ | $ | ( | ) | |||||
| Effects of exchange rate changes on cash and cash equivalents | $ | $ | $ | |||||||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
| Three months ended December 31, 2023 | ||||||||||||
| (unaudited) | As Reported | Adjustments | As Revised | |||||||||
| Cash flows from operating activities | ||||||||||||
| Inventories | $ | $ | $ | |||||||||
| Accounts payable | ( | ) | ||||||||||
| Other assets and liabilities | ( | ) | ||||||||||
| Net cash provided by operating activities | $ | $ | ( | ) | $ | |||||||
| Cash flows from investing activities | ||||||||||||
| Purchase of property, plant and equipment | $ | ( | ) | $ | $ | ( | ) | |||||
| Net cash provided by investing activities | $ | $ | $ | |||||||||
| Effects of exchange rate changes on cash and cash equivalents | $ | $ | $ | |||||||||
| Supplemental disclosures: | ||||||||||||
| Purchases of property, plant and equipment included in accounts payable and accrued expenses | $ | $ | $ | |||||||||
Item 9. Changes in and Disagreements with Accountants on Financial Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Exchange Act. Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to management, including the chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. Based upon this evaluation, our chief executive officer and our chief financial officer concluded that our disclosure controls and procedures were not effective as of September 30, 2024, the end of the period covered by this Annual Report on Form 10-K due to the material weakness described below.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act, as a process designed by, or under the supervision of our chief executive and chief financial officers and effected by our board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United Sates, or GAAP and includes those policies and procedures that:
| ● |
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and disposition of our assets; |
| ● |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and |
| ● |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an assessment of the effectiveness of our internal control over financial reporting as of September 30, 2024. In making this assessment, we used the criteria set forth in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations (COSO) of the Treadway Commission. Based on this evaluation, management concluded that, as of September 30, 2024, our internal control over financial reporting was not effective due to the material weakness described below.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company’s annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
We identified a material weakness in our internal control over financial reporting as we did not design and maintain effective controls related to the review of the cash flow statement. The material weakness resulted in immaterial misstatements in our Consolidated Statements of Cash Flows for the Q2 and Q3 interim periods during fiscal 2023, for the year ended September 30, 2023, as well as the Q1, Q2, and Q3 interim periods during fiscal 2024 and in our supplemental cash flow disclosures for the year ended September 30, 2022, each interim and annual period during fiscal 2023 and the Q1, Q2 and Q3 interim periods during fiscal 2024. Additionally, the material weakness could result in material misstatements of our interim or annual consolidated statement of cash flows or supplemental cash flow disclosures that would not be prevented or detected on a timely basis.
The effectiveness of our internal control over financial reporting as of September 30, 2024 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears in Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10-K.
Remediation Plan
Our management has taken, and plans to take, actions to remediate the deficiency in our internal control over financial reporting and will implement new processes, procedures and controls designed to address the underlying causes associated with the material weakness.
We are in the process of:
| i. |
implementing a new cash flow reporting tool which will automate the calculation of the effect of exchange rate changes on cash and cash equivalents, and |
| ii. |
implementing and documenting new processes and controls over the review of our consolidated statement of cash flows. |
We believe the measures described above will remediate the material weakness and strengthen our internal control over financial reporting. The material weakness will not be considered remediated until we have completed the design and implementation of the applicable controls and they operate for a sufficient period of time and management has concluded, through testing, that the controls are operating effectively. We are committed to continuing to improve our internal control over financial reporting, and as we continue to evaluate and work to improve our internal control over financial reporting, we may take additional measures beyond those remediation measures described above to address control deficiencies, or we may modify certain of the remediation measures described above.
Changes in Internal Control Over Financial Reporting
There were no changes in internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the fiscal fourth quarter ended September 30, 2024, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Rule 10b5-1 Trading Arrangements
During the three months ended September 30, 2024, director nor officer of the Company adopted, modified or terminated a Rule 10b5-1 trading arrangement or non-Rule 10b5-1 trading arrangement, as each term is defined in Item 408(a) of Regulation S-K, except:
On , , our , a Rule 10b5-1 trading arrangement for the period commencing three months from such date and ending on December 31, 2025 for the sale of up to shares of common stock of the Company.
Departure of Named Executive Officer
On July 16, 2024, Robin Vacha, the Company’s Senior Vice President, Global Operations, submitted his resignation effective August 16, 2024, and on such effective date, Mr. Vacha ceased to be employed by the Company.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
Item 10. Directors, Executive Officers and Corporate Governance
The information required by this Item 10 is contained in our definitive proxy statement for our 2025 annual meeting of stockholders to be filed by us within 120 days after the close of our fiscal year, or the 2024 Proxy Statement, under the captions “Proposal No. 1 Election of Directors,” “Other Matters-Delinquent Section 16(a) Reports,” if applicable, “Other Matters-Standards of Conduct,” “Other Matters-Stockholder Proposals and Recommendations for Director” and “Corporate Governance” and is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this Item 11 is contained under the captions “Corporate Governance,” “Compensation of Directors” and “Executive Officers” in the 2024 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item 12 is contained under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in the 2024 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item 13 is contained under the captions “Related Party Transactions,” “Corporate Governance” and “Compensation of Directors” in the 2024 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services
The information required by this Item 14 is contained under the caption “Independent Auditor Fees and Other Matters” in the 2024 Proxy Statement to be filed by us within 120 days after the close of our fiscal year and is incorporated herein by reference.
Item 15. Exhibits and Financial Statement Schedules
(a) Financial Statements and Financial Statement Schedules
| ● |
Consolidated Financial Statements of the Company and the related notes are included under Part II, Item 8, “Financial Statements and Supplementary Data” of this Annual Report on Form 10‑K. |
| ● |
Other financial statement schedules are omitted because of the absence of conditions under which they are required or because the required information is given in the supplementary Consolidated Financial Statements or notes thereto. |
(b) Exhibits
| Exhibit |
Description |
|
| 2.01* |
||
| 2.02 |
||
| 2.03* |
||
| 2.04*+ |
||
| 3.01 |
||
| 3.02 |
||
| 3.03 |
| 10.12** |
||
| 10.13** |
||
| 10.14** |
||
| 10.15 | Cooperation Agreement, by and among the Company and Politan Capital Management LP, Politan Capital Management GP LLC, Politan Capital NY LLC, and Politan Capital Partners GP LLC, dated as of November 1, 2024 (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, filed on November 4, 2024) | |
| 19 | Azenta, Inc. Insider Trading and Confidentiality of Insider Information Policy | |
| 21.01 |
||
| 23.01 |
||
| 31.01 |
||
| 31.02 |
||
| 32 |
||
| 97 | ||
| 101 |
The following material from the Company’s Annual Report on Form 10‑K, for the year ended September 30, 2024, formatted in iXBRL (Inline eXtensible Business Reporting Language): (i) the Consolidated Balance Sheets; (ii) the Consolidated Statements of Operations; (iii) the Consolidated Statements of Comprehensive Income (Loss); (iv) the Consolidated Statements of Cash Flows; (v) the Consolidated Statements of Changes in Stockholders’ Equity; and (vi) the Notes to Consolidated Financial Statements. The instance document does not appear in the Interactive Data File because XBRL tags are embedded in the iXBRL document. |
|
| 104 |
Cover Page Interactive Data File (formatted as iXBRL and contained in Exhibit 101). |
| * Certain schedules and exhibits have been omitted from this Exhibit pursuant to Item 601(a)(5) of Regulation S-K. Azenta, Inc. will furnish a copy of any omitted schedule or exhibit to the U.S. Securities and Exchange Commission or its staff upon request. |
| ** Management contract, compensatory plan or agreement. |
| + Certain confidential portions (indicated by brackets and asterisk) have been omitted from this Exhibit. |
None.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AZENTA, INC. |
|||
| By: |
/S/ JOHN MAROTTA |
||
| John Marotta |
|||
Date: November 26, 2024
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date |
| /S/ JOHN MAROTTA |
Director, President and Chief Executive Officer |
November 26, 2024 |
| John Marotta |
(Principal Executive Officer) | |
| /S/ HERMAN CUETO |
Executive Vice President and |
November 26, 2024 |
| Herman Cueto | Chief Financial Officer | |
| (Principal Financial Officer) | ||
| /S/ VIOLETTA A. HUGHES |
Vice President and |
November 26, 2024 |
| Violetta A. Hughes |
Chief Accounting Officer | |
| (Principal Accounting Officer) | ||
| /S/ FRANK E. CASAL |
Director |
November 26, 2024 |
| Frank E. Casal |
||
| /S/ ROBYN C. DAVIS |
Director |
November 26, 2024 |
| Robyn C. Davis |
||
| /S/ EDWARD P. BOUSA |
Director |
November 26, 2024 |
| Edward P. Bousa |
||
| /S/ ERICA J. MCLAUGHLIN |
Director |
November 26, 2024 |
| Erica J. McLaughlin |
||
| /S/ TINA S. NOVA |
Director |
November 26, 2024 |
| Tina S. Nova |
||
| /S/ DIDIER HIRSCH |
Director |
November 26, 2024 |
| Didier Hirsch | ||
| /S/ MARTIN D. MADAUS |
Director |
November 26, 2024 |
| Martin D. Madaus |
||
| /S/ MICHAEL ROSENBLATT |
Director |
November 26, 2024 |
| Michael Rosenblatt |
||
| /S/ WILLIAM L. CORNOG | Director |
November 26, 2024 |
| William L. Cornog | ||
| /S/ QUENTIN KOFFEY | Director | November 26, 2024 |
| Quentin Koffey | ||
| /S/ ALAN J. MALUS | Director |
November 26, 2024 |
| Alan J. Malus |