No Data
02179 RECBIO-B
- 8.280
- 0.0000.00%
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Capital Trend
No Data
News
The U.S. bioterrorism law has undergone significant changes, the CRO concept has launched a major counterattack, and WuXi AppTec is excited!
Industry pessimistic expectations may improve.
How to break through the challenges in the development of innovative drugs? The industry suggests focusing on internationalization.
① The year 2024 will be the inaugural year of large-scale authorized trade, and going abroad has become an important direction for the development of local pharmaceutical companies; ② Chinese企业品牌, clinical trial capabilities, data presentation formats, and levels of international operation still need time and practical verification.
Jiangsu Recbio Technology Shareholders to Vote Dec. 24 on Share Issue
Express News | Jiangsu Recbio Technology - to Issue Not More Than 143.1 Mln Domestic Shares at RMB5.59 per Share
Jiangsu Recbio Technology Initiates Phase III Clinical Trials for Shingles Vaccine
RECBIO-B: 2024 Interim Report
Comments
Hong Kong-listed vaccine makers, buoyed by the news, broadly shot up. $CANSINOBIO (06185.HK)$rose 6.6% to $90.6, on a volume of 9.0554 million shares, involving $801 million. $RECBIO-B (02179.HK)$ , $INNOCARE (09969.HK)$ and �����...
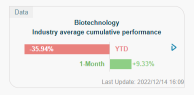



whqqq : Will they fall after a pandemic?
Spinee OP whqqq : It's hard to say![undefined [undefined]](https://static.moomoo.com/nnq/emoji/static/image/default/default-black.png?imageMogr2/thumbnail/36x36)
whqqq Spinee OP : But it can still be held for a short time, right?![undefined [undefined]](https://static.moomoo.com/nnq/emoji/static/image/default/default-black.png?imageMogr2/thumbnail/36x36)