No Data
06628 TRANSCENTA-B
- 0.700
- -0.030-4.11%
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
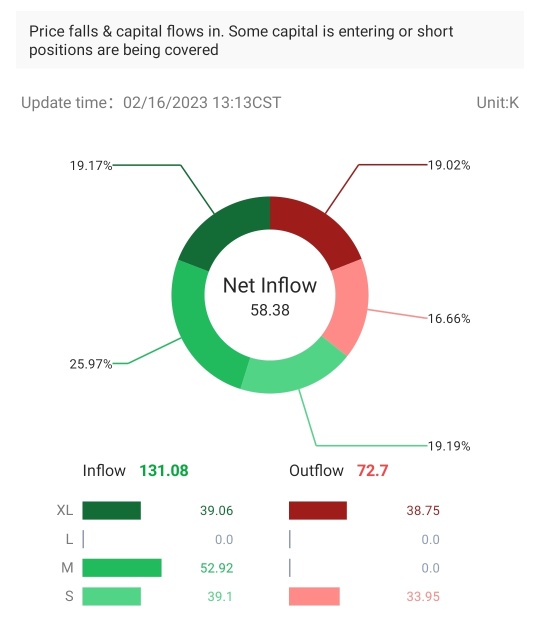
Trade Overview
Capital Trend
No Data
News
Chuangsheng Group-B (06628.HK) has seen the executive director, chairman and CEO Qian Xueming increase shareholding by 0.15 million shares.
According to the latest equity disclosure information from the Hong Kong Stock Exchange on December 2, Glorious Resources (06628.HK) was acquired by executive director, chairman, and CEO Qian Xueming in the market on November 27-28, 2024, with an average price per share of HK$0.61-0.64, totaling 0.15 million shares, involving approximately HK$0.093 million. After the increase, Qian Xueming's latest shareholding number is 37.3505 million shares, and the shareholding ratio has risen from 8.52% to 8.56%.
On November 27, Chuangsheng Group-B (06628.HK) spent 0.0499 million Hong Kong dollars to repurchase 0.078 million shares.
Gelonghui November 27丨Chuangsheng Group-B (06628.HK) announced that on November 27, it spent 0.0499 million Hong Kong dollars to repurchase 0.078 million shares.
Chuangsheng Group - B (06628) spent approximately 0.0112 million Hong Kong dollars to repurchase 0.0145 million shares on November 21.
Chuangsheng Group-B (06628) announced that on November 21, 2024, approximately 0.0112 million Hong Kong dollars were spent to repurchase 1....
Chuangsheng Group - B (06628.HK) spent 0.05 million Hong Kong dollars to repurchase 0.064 million shares on November 20th.
Gelonghui, November 20丨Chuangsheng Group-B (06628.HK) announced that on November 20, it spent 0.05 million Hong Kong dollars to repurchase 0.064 million shares.
Chuang Sheng Group -B (06628.HK) spent 0.049 million Hong Kong dollars to repurchase 0.059 million shares on November 19th.
Gelonghui, November 19丨Chuangsheng Group-B (06628.HK) announced that on November 19, it spent 0.049 million HKD to repurchase 0.059 million shares, with a repurchase price of 0.83 HKD per share.
Chun Sheng Group-B (06628.HK): Announced at the ESMO 2024 Congress, the latest business progress of OSEMITAMAB (TST001) triplet therapy for the first-line treatment of inspiring efficacy data for gastric or gastroesophageal junction adenocarcinoma.
On September 19th, Changsheng Group-B (06628.HK) announced updated research data on the G-queue for the first-line treatment of advanced gastric or gastroesophageal junction adenocarcinoma (TranStar102) using Osemitamab (TST001) in combination with Narulitinib and CAPOX. The data from this queue has consistently shown encouraging efficacy since its announcement at the ASCO 2024 conference. The study results showed that in patients with known levels of CLDN18.2 and PD-L1 expression, patients with high/medium expression of CLDN18.2 had a median progression-free survival (mPFS).
Comments
Analysis
Price Target
No Data
No Data