No Data
US Stock MarketDetailed Quotes
AGIO Agios Pharmaceuticals
- 35.900
- +0.780+2.22%
Close Jan 8 16:00 ET
- 35.900
- 0.0000.00%
Post 16:45 ET
2.05BMarket Cap3.08P/E (TTM)
36.000High34.470Low1.03MVolume35.140Open35.120Pre Close36.72MTurnover2.04%Turnover RatioLossP/E (Static)57.03MShares62.58052wk High1.26P/B1.81BFloat Cap20.96052wk Low--Dividend TTM50.52MShs Float138.850Historical High--Div YieldTTM4.36%Amplitude15.770Historical Low35.554Avg Price1Lot Size
Full Hours
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Agios' Supplemental New Drug Application for Its Thalassemia Treatment Gets FDA Nod
Cardurion Pharmaceuticals Appoints Charlotte Newman as Chief Business Officer
Agios Pharmaceuticals Says FDA Accepted SNDA For PYRUKYND (Mitapivat) For Treatment Of Adult Patients With Non-Transfusion-Dependent And Transfusion-Dependent Alpha- Or Beta-thalassemia
Express News | Agios Pharmaceuticals Inc - Pdufa Goal Date for Pyrukynd Snda Is September 7, 2025
FDA Accepts Agios' Supplemental New Drug Application for PYRUKYND (Mitapivat) in Adult Patients With Non-Transfusion-Dependent and Transfusion-Dependent Alpha- or Beta-Thalassemia
BofA Securities Maintains Agios Pharmaceuticals(AGIO.US) With Buy Rating, Announces Target Price $55
Comments
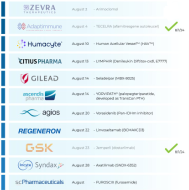
🚨 This Week’s PDUFA:
$Humacyte (HUMA.US)$ : 🤔
⇨ Human Acellular Vessel (HAV)™
‣ Vascular Trauma
‣ PDUFA: 8/10/24 (BLA)
🗓️ Last Week’s AdCom & PDUFAs:
$Zevra Therapeutics (ZVRA.US)$ : Favorable AdCom 👍
🗳️ 11-5 votes in favor
⇨ Arimoclomol
‣ Niemann-Pick disease type C
‣ AdCom: 8/2/24 (NDA)
$Adaptimmune Therapeutics (ADAP.US)$ : Approved 🎉
⇨ TECELRA (afamitresgene autoleucel)
‣ Synovial sarcoma
‣ PDUFA: 8/4/24 (BLA)
$GlaxoSmithKline (GSK.US)$ : Approved 🎉
⇨ Jemperli
‣ Endometria...
$Humacyte (HUMA.US)$ : 🤔
⇨ Human Acellular Vessel (HAV)™
‣ Vascular Trauma
‣ PDUFA: 8/10/24 (BLA)
🗓️ Last Week’s AdCom & PDUFAs:
$Zevra Therapeutics (ZVRA.US)$ : Favorable AdCom 👍
🗳️ 11-5 votes in favor
⇨ Arimoclomol
‣ Niemann-Pick disease type C
‣ AdCom: 8/2/24 (NDA)
$Adaptimmune Therapeutics (ADAP.US)$ : Approved 🎉
⇨ TECELRA (afamitresgene autoleucel)
‣ Synovial sarcoma
‣ PDUFA: 8/4/24 (BLA)
$GlaxoSmithKline (GSK.US)$ : Approved 🎉
⇨ Jemperli
‣ Endometria...
6
$Agios Pharmaceuticals (AGIO.US)$
HOW MANY STUPID TRADERS HERE, LOSING AND STILL TRADING 🤔🤔🤔
HOW MANY STUPID TRADERS HERE, LOSING AND STILL TRADING 🤔🤔🤔
$Agios Pharmaceuticals (AGIO.US)$
Bad Demon Trap = Xi Zor Gai 😏
Bad Demon Trap = Xi Zor Gai 😏
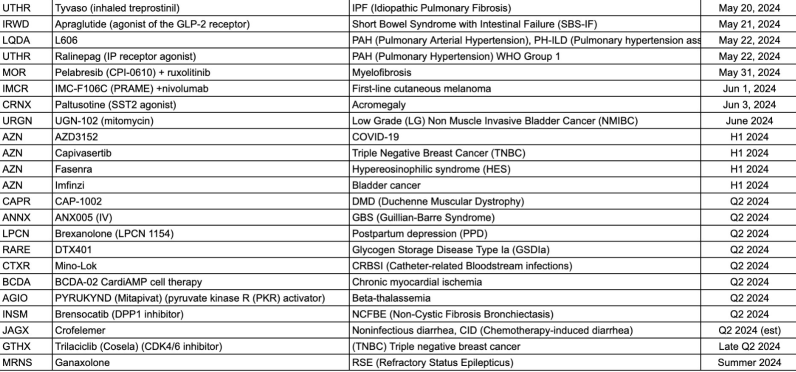
$United Therapeutics (UTHR.US)$ Phase 3
$Ironwood Pharmaceuticals (IRWD.US)$ Phase 3
$Liquidia (LQDA.US)$ Phase 3
$United Therapeutics (UTHR.US)$ Phase 3
$MorphoSys (MOR.US)$ Phase 3
$Immunocore (IMCR.US)$ Phase 3
$Crinetics (CRNX.US)$ Phase 3
$UroGen Pharma Ltd (URGN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$Capricor Therapeutics (CAPR.US)$ Phase 3
$Annexon (ANNX.US)$ Phase 3
$Lipocine (LPCN.US)$ Phase 3�...
$Ironwood Pharmaceuticals (IRWD.US)$ Phase 3
$Liquidia (LQDA.US)$ Phase 3
$United Therapeutics (UTHR.US)$ Phase 3
$MorphoSys (MOR.US)$ Phase 3
$Immunocore (IMCR.US)$ Phase 3
$Crinetics (CRNX.US)$ Phase 3
$UroGen Pharma Ltd (URGN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$AstraZeneca (AZN.US)$ Phase 3
$Capricor Therapeutics (CAPR.US)$ Phase 3
$Annexon (ANNX.US)$ Phase 3
$Lipocine (LPCN.US)$ Phase 3�...
19
2
2
Despite the recent upturn, the company’s financial performance is seen as too risky due to its sustained revenue decline and loss-making status.
1
Read more
Trending US Stocks

Fed Rate Cut Beneficiaries Fed Rate Cut Beneficiaries
Stocks that are expected to benefit from a Federal Reserve rate cut. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in Fed Rate Cut Beneficiaries, ranked from highest to lowest based on real-time market data. Stocks that are expected to benefit from a Federal Reserve rate cut. Information is provided by Futu and is a non-exhaustive list of all thematic stocks for reference purposes only.
This section presents the top 5 stocks in Fed Rate Cut Beneficiaries, ranked from highest to lowest based on real-time market data.



