No Data
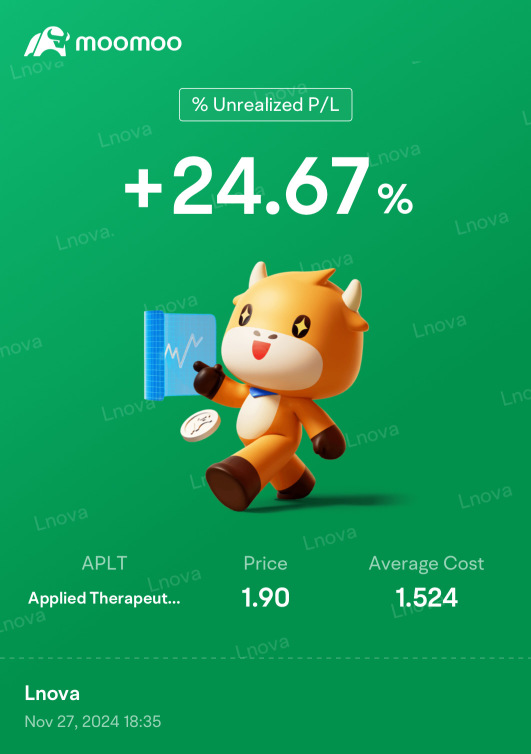
APLT Applied Therapeutics
- 8.570
- -1.640-16.06%
- 1.950
- -6.620-77.25%
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Capital Trend
No Data
News
Wait for Galactosemia Therapy Goes on After FDA Shuns Applied Therapeutics' Govorestat
Outlook OTLK -65% Phase 3 Fail; Applied Therapeutics APLT Halted AH CRL
Applied Therapeutics Receives Complete Response Letter From U.S. FDA Regarding New Drug Application For Govorestat For Classic Galactosemia
FDA rejected the drug Applied Therapeutics in a complete response letter, causing a 78% plunge in after-hours trading.
On Wednesday, $applied therapeutics(APLT.us)$ plummeted by 78% after hours to $1.89. On the news front, the US FDA has rejected the company's application for govorestat to treat the rare metabolic disease classic galactosemia. In a comprehensive response letter, the FDA stated that due to deficiencies in the application, it could not approve the drug. The company stated it is reviewing the FDA's feedback and will request a meeting with the institutions to discuss the necessary conditions for a potential resubmission. Govorestat is currently targeting sorbitol dehydrogenase.
Trending Stocks Today | Biomerica Surges 36.1% Post-Market
Applied Therapeutics Down 76% After Hours on FDA Complete Response Letter
Comments
Analysis
Price Target
No Data
No Data

 prediction for what? For this stock? I don’t know what will happen. Am a newbie
prediction for what? For this stock? I don’t know what will happen. Am a newbie
