No Data
AZN241129C77000
- 0.09
- 0.000.00%
- 5D
- Daily
News
Astrazeneca (AZN.US) combination therapy Truqap phase 3 trial results are positive.
Astrazeneca (AZN.US) announced today the positive results of the CAPItello-281 Phase 3 clinical trial.
Astrazeneca's Capivasertib for prostate cancer has successfully completed Phase III trials, and the outlook for Kairui Pharmaceutical-B (2105.HK) AKT inhibitors is bullish.
On November 25th, AstraZeneca announced the success of its AKT inhibitor Capivasertib in the phase III CAPItello-281 study for the treatment of newly diagnosed metastatic hormone-sensitive prostate cancer (mHSPC). Following the approval and launch of Capivasertib for breast cancer indications, this study further confirmed the clinical value of AKT inhibitors. AKT inhibitors, as drugs that have shown potential in multiple cancers such as breast cancer, prostate cancer, and ovarian cancer, have a very broad market outlook. In recent years, an increasing number of pharmaceutical companies have begun to pay attention to AKT inhibitors.
AstraZeneca Reports Positive Phase 3 Results for Truqap in Prostate Cancer
AstraZeneca (AZN) Receives a Buy From Goldman Sachs
TRUQAP (Capivasertib) Combination in PTEN-deficient Metastatic Hormone-sensitive Prostate Cancer Demonstrated Statistically Significant and Clinically Meaningful Improvement in Radiographic Progression-free Survival in CAPItello-281 Phase III Trial
AstraZeneca's Truqap Combo Demonstrated Statistically Significant Improvement
Comments
$Applied Therapeutics (APLT.US)$ : 🤔
⇨ Govorestat
‣ Classic Galactosemia
‣ PDUFA: 11/28/24 (NDA)
🗓️ Last Week’s Adcom & PDUFA decisions:
$BridgeBio Pharma (BBIO.US)$ : Approved 11/22 ✅
⇨Attruby (Acoramidis)
‣ ATTR-CM (transthyretin amyloid cardiomyopathy)
‣ PDUFA: 11/29/24 (NDA)
$AstraZeneca (AZN.US)$ : Discussion, no vote
⇨ Andexxa (coagulation factor Xa)
‣ Life-threatening or uncontrolled bleeding
‣ Adcom: 11/21/24 (sBLA)
$Jazz Pharmaceuticals (JAZZ.US)$ & $Zymeworks (ZYME.US)$ : Approve...

New Positions
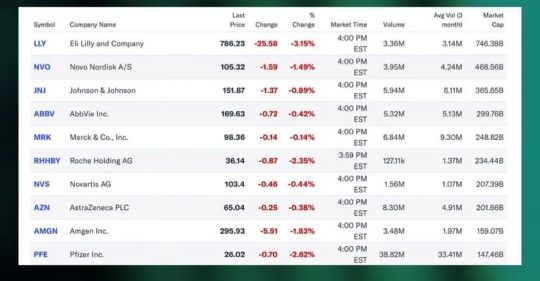
$Taiwan Semiconductor (TSM.US)$ , $Broadcom (AVGO.US)$ , $Johnson & Johnson (JNJ.US)$ , $Pfizer (PFE.US)$ , $Coca-Cola (KO.US)$ , $Occidental Petroleum (OXY.US)$
Sold out Positions
$Disney (DIS.US)$
Increased Positions
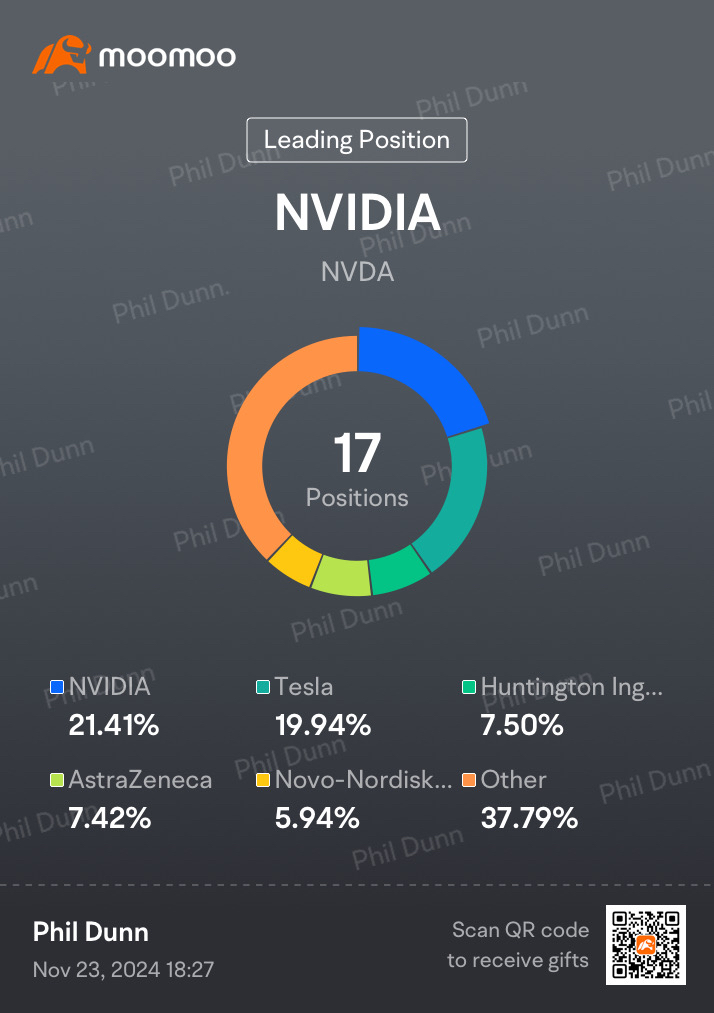
$Novo-Nordisk A/S (NVO.US)$ , $AstraZeneca (AZN.US)$ , $Huntington Ingalls Industries (HII.US)$
Decreased Positions
None
loading...
Gapping up
$Comcast (CMCSA.US)$ stock increased by 2.5% following a report by the WSJ that the media giant was nearing approval for a $7 billion spinoff of its cable TV assets.
$NVIDIA (NVDA.US)$ stock went up by 0.44%, building on the near 5% gains from the previous session as the market anticipates its upcoming quarterly earnings report.
$Keysight Technologies (KEYS.US)$ stock jumped 9.4% after the company rel...
Hugely Undervalued $2 Biotech With 21% Held Short $16 Price Target, Takeover Candidate, Soon Profitable. Fund that owns $Viking Therapeutics (VKTX.US)$ since 2022 below $10 a share is also investing in ESPR and expects the company will be a block buster.
This unique biotech $Esperion Therapeutics (ESPR.US)$ Esper...