No Data
BGNE BeiGene
- 181.890
- -2.790-1.51%
- 181.890
- 0.0000.00%
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Capital Trend
No Data
News
Exchange-Traded Funds, Equity Futures Lower Pre-Bell Friday as Markets Sluggishly Move to Year-End
BeiGene Gains FDA Nod for TEVIMBRA, Marking Key Progress in Oncology Treatments
TEVIMBRA Approved in U.S. for First-line Treatment of Gastric and Gastroesophageal Junction Cancers in Combination With Chemotherapy
Express News | Tevimbra Approved in U.S. for First-Line Treatment of Gastric and Gastroesophageal Junction Cancers in Combination With Chemotherapy
Kaiyuan Securities: Each subtype of hematological tumors has the potential to hatch "heavyweight bomb" drugs. Pay attention to Innovative Drugs companies.
The subtype of blood tumors is less aggressive with a longer treatment cycle, generally requiring long-term medication. Patients have relatively high viscosity and the potential for the emergence of "blockbuster" drugs.
Highly resilient! Leading CRO companies have stabilized their fundamentals, but the shadow of the biosafety bill may still linger next year | Year-end review.
① In 2024, the USA Biodefense Act will disrupt CROs throughout the year and may continue next year; ② In the first three quarters of this year, over half of listed companies experienced revenue growth, and leading CROs demonstrated resilience; ③ Research on popular targets such as ADC, GLP-1, and bispecific antibodies will continue to be hot; ④ In 2025, the performance of leading CROs and small to medium enterprises may further diverge.
Comments
$Ionis Pharmaceuticals (IONS.US)$ : 🤔
⇨ IONIS-APOCIII-LRx (Olezarsen)
‣ FCS (Familial chylomicronemia syndrome)
‣ PDUFA: 12/19/24 (NDA)
🗓️ Last Week’s PDUFA Decisions:
$Checkpoint Therapeutics (CKPT.US)$ : Approved 12/13/24 ✅
⇨ UNLOXCYT (cosibelimab-ipdl)
‣ metastatic or locally advanced cSCC (cutaneous squamous cell carcinoma)
‣ PDUFA: 12/28/24 (resubmitted BLA)
$Neurocrine Biosciences (NBIX.US)$ : Approved 12/13/24 ✅
⇨ CRENESSITY™ (crin...
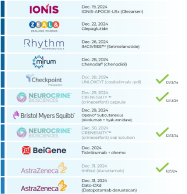
$Ionis Pharmaceuticals (IONS.US)$ ⇨ IONIS-APOCIII-LRx (Olezarsen)
$ZLDPF ⇨ Glepaglutide
$Rhythm Pharmaceuticals (RYTM.US)$ ⇨ IMCIVREE™ (Setmelanotide)
$Mirum Pharmaceuticals (MIRM.US)$ ⇨ Chenodal® (chenodiol)
$Checkpoint Therapeutics (CKPT.US)$ ⇨ Cosibelimab (CK-301)
$Neurocrine Biosciences (NBIX.US)$ ⇨ Crinecerfont capsule
$Bristol-Myers Squibb (BMY.US)$ ⇨ Opdivo® Subcutaneous (nivolumab + hyaluronidase)
$Neurocrine Biosciences (NBIX.US)$ ⇨ Crinecerfont oral solution
$BeiGene (BGNE.US)$ ⇨ Tisleli...
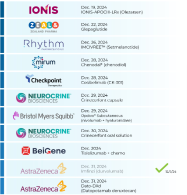
$Ionis Pharmaceuticals (IONS.US)$ ⇨ IONIS-APOCIII-LRx (Olezarsen)
$ZLDPF ⇨ Glepaglutide
$Rhythm Pharmaceuticals (RYTM.US)$ ⇨ IMCIVREE™ (Setmelanotide)
$Mirum Pharmaceuticals (MIRM.US)$ ⇨ Chenodal® (chenodiol)
$Checkpoint Therapeutics (CKPT.US)$ ⇨ Cosibelimab (CK-301)
$Neurocrine Biosciences (NBIX.US)$ ⇨ Crinecerfont capsule
$Bristol-Myers Squibb (BMY.US)$ ⇨ Opdivo® Subcutaneous (nivolumab + hyaluronidase)
$Neurocrine Biosciences (NBIX.US)$ ⇨ Crinece...
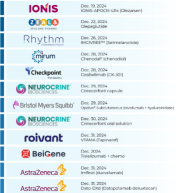
$Heron Therapeutics (HRTX.US)$ : 🤔
⇨ ZYNRELEF® Vial Access Needle
‣ Postoperative pain
‣ PDUFA: 9/23/24 (sNDA)
$Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ KarXT (xanomeline-trospium)
‣ Schizophrenia
‣ PDUFA: 9/26/24 (NDA)
$Sanofi (SNY.US)$ $Regeneron Pharmaceuticals (REGN.US)$ : 🤔
⇨ Dupixent
‣ Chronic obstructive pulmonary disease
‣ PDUFA: 9/27/24 (sBLA)
$BeiGene (BGNE.US)$ $Merck & Co (MRK.US)$ & $Bristol-Myers Squibb (BMY.US)$ : 🤔
⇨ Tislelizumab, KEYTRUDA, OPDIVO & YER...



Just Care Bears : I thought there’s a FDA approval going on on this today no news yet
Claudius NJIE Just Care Bears : yes good news is coming.
Claudius NJIE : pls run to at least 50