No Data
US Stock MarketDetailed Quotes
BHC Bausch Health
- 7.810
- +0.090+1.17%
Close Jan 23 16:00 ET
- 7.810
- 0.0000.00%
Post 20:01 ET
2.87BMarket Cap-16.27P/E (TTM)
7.995High7.730Low1.57MVolume7.820Open7.720Pre Close12.37MTurnover0.57%Turnover RatioLossP/E (Static)367.80MShares11.46052wk High-2.45P/B2.13BFloat Cap3.96052wk Low--Dividend TTM273.17MShs Float34.800Historical High--Div YieldTTM3.43%Amplitude3.960Historical Low7.887Avg Price1Lot Size
Full Hours
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Trade Overview
Unit: --
Capital Trend
IntradayDayWeekMonth
No Data
News
Amneal Pharmaceuticals Gets US FDA Approvals for Alzheimer's, Oncology Treatments, Gastrointestinal Disease
Bausch Health to Announce Fourth Quarter and Full Year 2024 Results on February 19, 2025
Market Chatter: Bausch Health Swaps Out Advisers After Years of Debt, Sale Talks
RBC on FDA's Third Tentative Approval For Rifaximin 550mg to Amneal Pharmaceuticals
Bausch Health And Salix Pharmaceuticals Announced That CMS Selected XIFAXAN 550 Mg For Drug Price Negotiation Under Inflation Reduction Act, Effective 2027; XIFAXAN Approved For HE And IBS-D In Adults, Rated Grade I, A,1 By Liver Disease Associations
Bausch Health Statement on Selection of XIFAXAN(R) (Rifaximin) for Inflation Reduction Act's Medicare Negotiation Program
Comments
Bausch Health Responds to Market Rumors.
Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) recently found itself at the center of market rumors that suggested the company was considering bankruptcy or insolvency proceedings. These rumors originated from an article issued by Reorg®, citing unnamed sources. In response, Bausch Health has categorically denied these claims, emphasizing that they are unsubstantiated and without merit...
Bausch Health Companies Inc. (NYSE:BHC)(TSX:BHC) recently found itself at the center of market rumors that suggested the company was considering bankruptcy or insolvency proceedings. These rumors originated from an article issued by Reorg®, citing unnamed sources. In response, Bausch Health has categorically denied these claims, emphasizing that they are unsubstantiated and without merit...
Bausch Health's revenue growth is unimpressive due to its lack of profitability. The share price crash may be an over-reaction, but improvements in business fundamentals are needed before considering a purchase. The company's long-term performance is concerning, and clear growth indicators are required for share price stability.
2
News Highlights
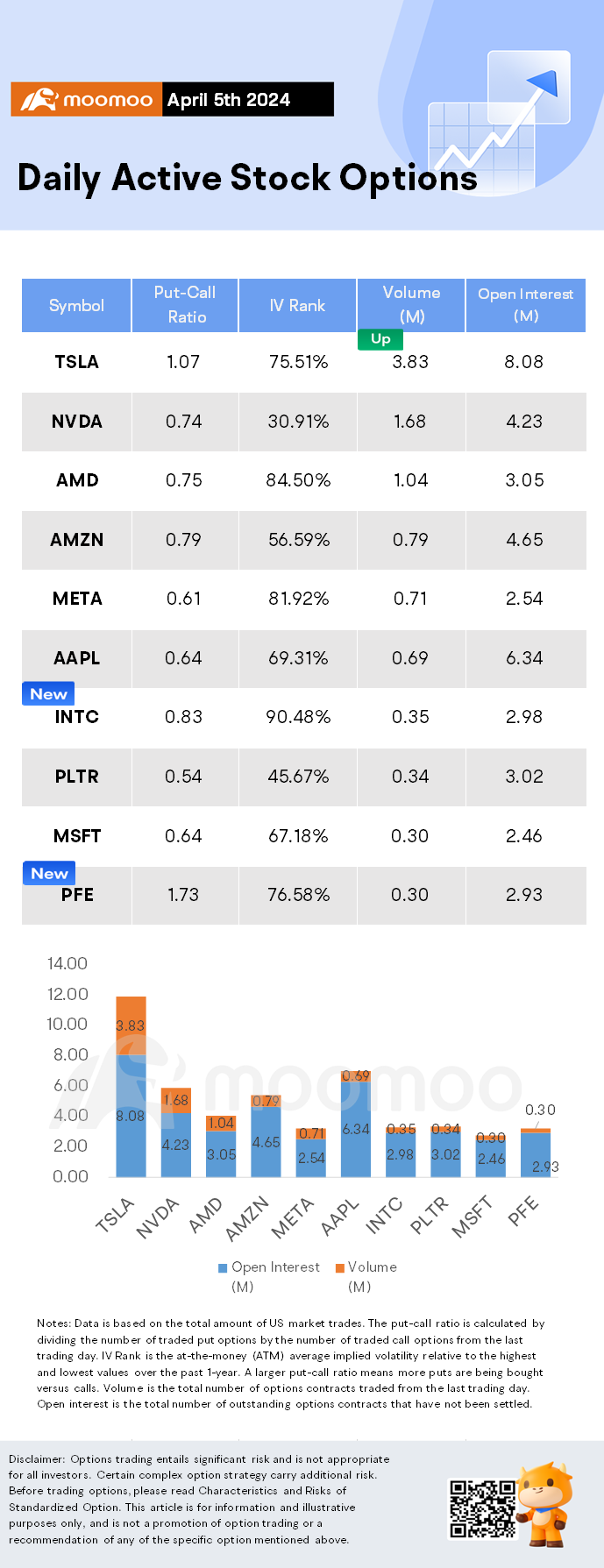
$Tesla (TSLA.US)$ shares fell by 3.63%, closing at $164.90. Its options trading volume was 3.83 million. Call contracts account for 48.3% of the total trading volume. The most traded calls are contracts of $170 strike price that expire on Apr. 5th. The total volume reaches 106,470 with an open interest of 29,026. The most traded puts are contracts of a $165 strike p...
$Tesla (TSLA.US)$ shares fell by 3.63%, closing at $164.90. Its options trading volume was 3.83 million. Call contracts account for 48.3% of the total trading volume. The most traded calls are contracts of $170 strike price that expire on Apr. 5th. The total volume reaches 106,470 with an open interest of 29,026. The most traded puts are contracts of a $165 strike p...
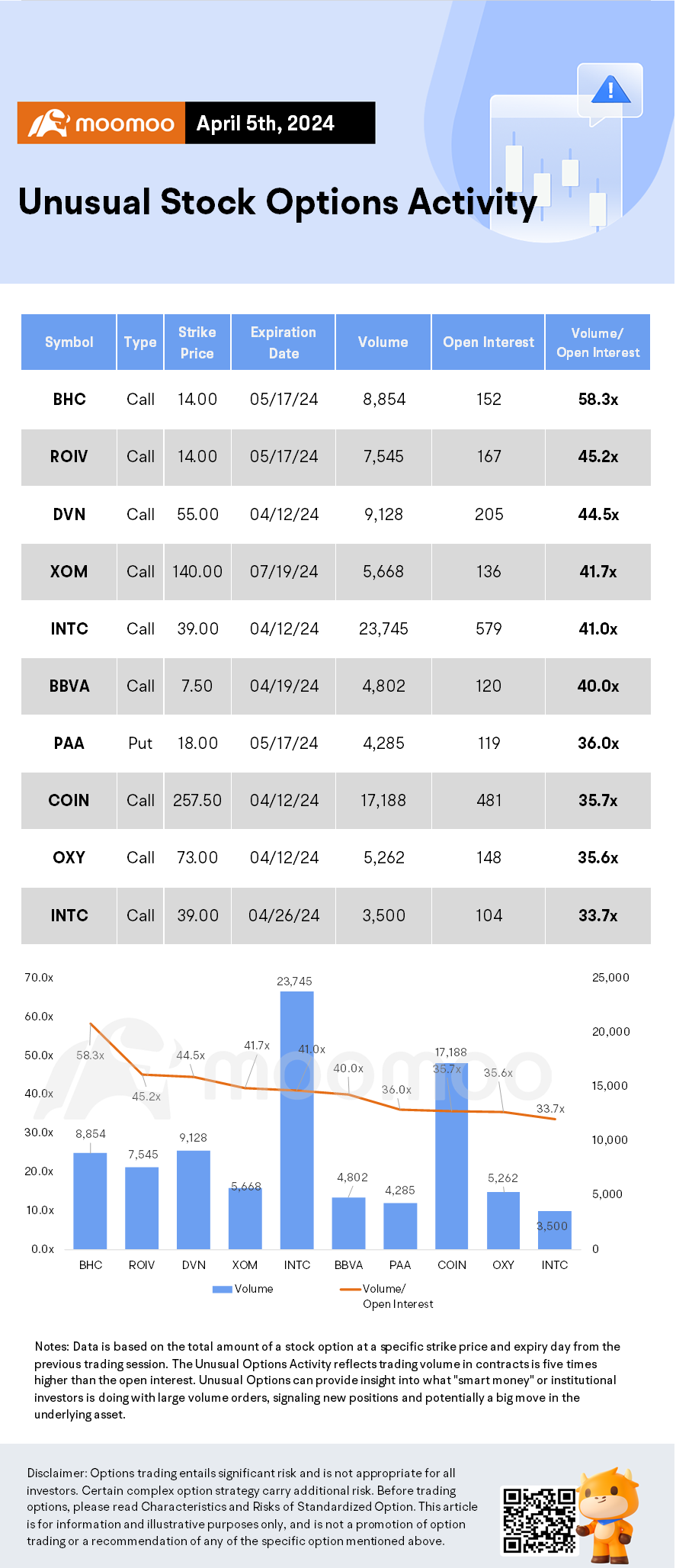
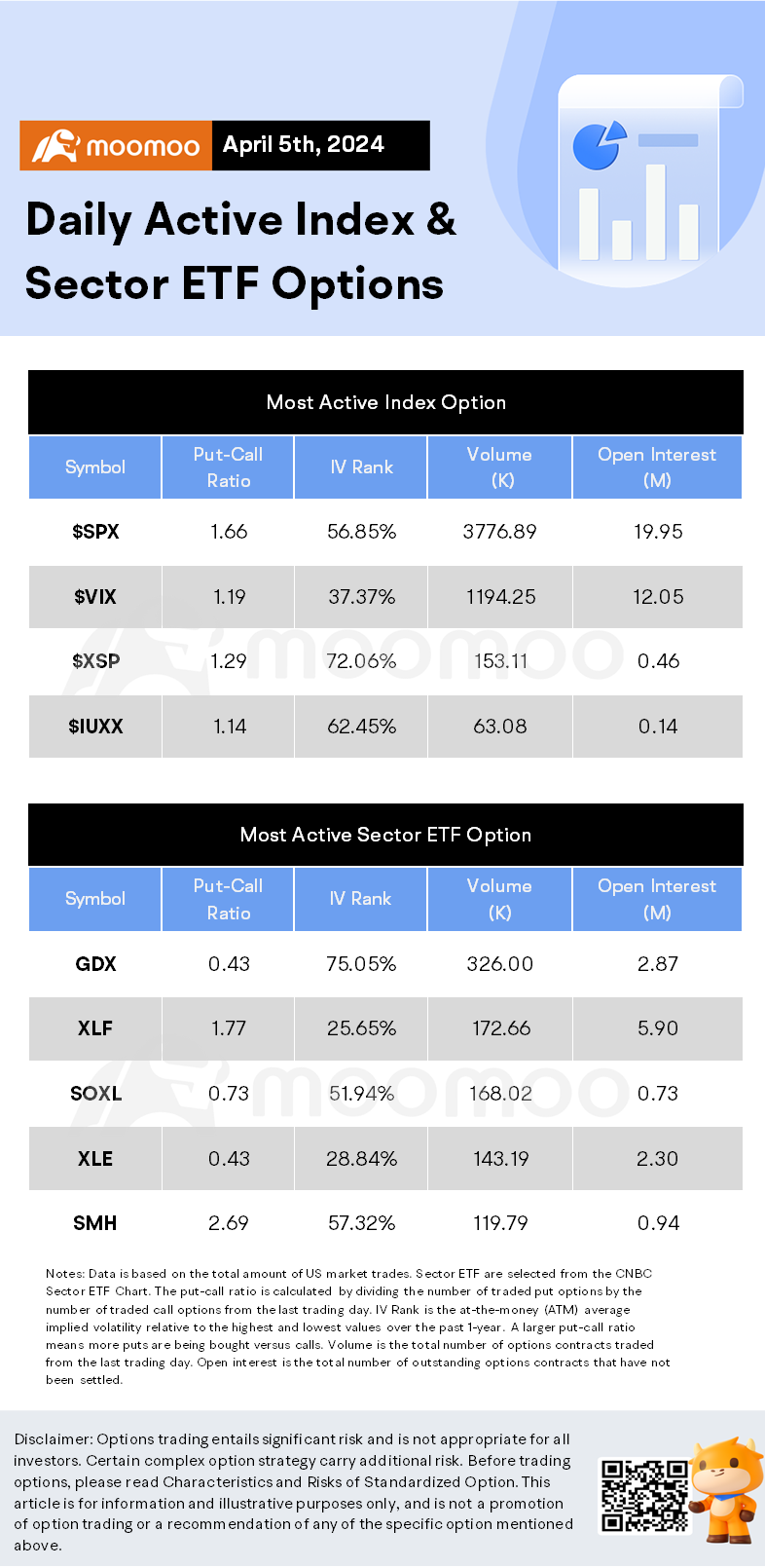
45
24
48
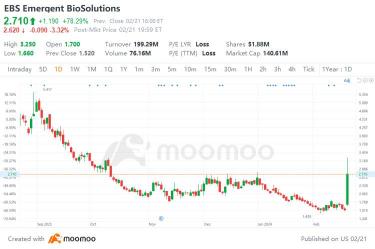
In today’s market, one of the biggest winners generating outsized interest is $Emergent BioSolutions (EBS.US)$ . This mid-cap biotech company remains a unique turnaround story for investors, and many appear to be placing their bets on this company to come out of the pile as a key winner. Indeed, EBS stock has surged over 70% higher on some intriguing news this afternoon.
The company’s turnaround efforts appear to be receiving a jolt today, wit...
The company’s turnaround efforts appear to be receiving a jolt today, wit...
8
1
1
Read more


