Biotechnology
- 4289.098
- +49.785+1.17%
Close Nov 27 16:00 ET
4296.689High4250.079Low
4262.293Open4239.312Pre Close653.10MVolume437RiseLossP/E (Static)6.93BTurnover36Flatline2.13%Turnover Ratio923.51BMarket Cap220Fall748.30BFloat Cap
Intraday
- 5D
- Daily
- Weekly
- Monthly
- 1Q
- 1Y
Constituent Stocks
SymbolStock Name
Latest PriceChg% ChgVolumeTurnoverOpenPre CloseHighLowMarket CapFloat CapSharesShs Float5D % Chg10D % Chg20D % Chg60D % Chg120D % Chg250D % Chg% Year-to-dateDiv YieldTTMTurnover RatioP/E (TTM)P/E (Static)AmplitudeIndustry
WatchlistPaper Trade
CYCNCyclerion Therapeutics
2.16000.8000+58.82%29.94M64.39M2.15001.36002.34001.72005.85M3.08M2.71M1.42M+3.35%+20.00%-28.00%-27.74%-15.29%-0.92%-35.52%--2101.93%LossLoss45.59%Biotechnology
HURATuHURA Biosciences
5.0501.560+44.70%3.46M17.03M4.0003.4905.6503.960213.54M134.68M42.28M26.67M+53.50%+2.23%-17.08%-17.69%-31.39%-57.32%-14.93%--12.96%LossLoss48.42%Biotechnology
CEROCERo Therapeutics
0.2020.055+37.62%64.12M13.34M0.1500.1470.2550.15030.41M14.16M150.31M70.00M-18.69%-4.53%+148.22%+71.30%-69.95%-98.14%-98.16%--91.60%LossLoss71.43%Biotechnology
CABACabaletta Bio
3.0100.590+24.38%4.57M12.95M2.6002.4203.0502.480147.12M125.11M48.88M41.57M+33.19%-24.94%-20.58%-40.51%-71.44%-81.25%-86.74%--11.01%LossLoss23.55%Biotechnology
ARWRArrowhead Pharmaceuticals
26.1505.100+24.23%6.69M168.25M22.37021.05026.62022.2503.25B2.89B124.43M110.53M+39.76%+20.90%+32.67%+9.05%+9.37%+23.35%-14.54%--6.06%LossLoss20.76%Biotechnology
PPBTPurple Biotech
3.2500.610+23.11%209.90K665.02K2.7302.6403.6502.5605.09M4.34M1.57M1.34M+31.58%+22.18%-1.52%-63.89%-71.59%-84.38%-79.17%--15.72%0.44Loss41.29%Biotechnology
FRESFresh2
1.3140.242+22.59%12.07K15.11K1.1181.0721.3191.1183.22M2.54M2.45M1.93M+8.61%+30.12%-16.82%-32.95%-69.97%-87.12%-85.23%--0.62%LossLoss18.79%Biotechnology
IPSCCentury Therapeutics
1.6300.280+20.74%1.26M1.93M1.3601.3501.6301.355138.60M63.12M85.03M38.72M+29.37%+22.56%+27.34%+2.52%-44.56%+18.98%-50.90%--3.27%LossLoss20.37%Biotechnology
TCRTAlaunos Therapeutics
2.81000.4800+20.60%51.72K137.05K2.33002.33002.88002.33004.50M3.68M1.60M1.31M+31.92%+17.08%+39.12%+11.95%-71.90%-71.70%-73.50%--3.95%LossLoss23.61%Biotechnology
IMMXImmix Biopharma
1.9700.320+19.39%700.19K1.40M1.6501.6502.2001.60054.19M31.45M27.51M15.96M+8.24%+25.48%+19.39%-2.14%-2.96%-61.45%-71.53%--4.39%LossLoss36.36%Biotechnology
FBRXForte Biosciences
19.7903.110+18.65%278.53K5.69M17.50016.68023.36416.86028.93M17.74M1.46M896.39K+46.05%+249.03%+326.51%+171.10%+35.76%+67.00%-3.66%--31.07%LossLoss38.99%Biotechnology
FATEFate Therapeutics
3.1700.490+18.28%5.89M18.21M2.6802.6803.2702.680361.05M313.16M113.89M98.79M+42.79%+46.08%+27.31%-9.94%-13.86%+27.31%-15.24%--5.96%LossLoss22.02%Biotechnology
RENBRenovaro
0.6100.093+18.07%603.21K348.07K0.5170.5170.6200.51796.87M47.25M158.72M77.42M+21.14%+1.38%+13.02%+17.59%-52.69%-81.67%-80.75%--0.78%LossLoss19.95%Biotechnology
IMNMImmunome
13.4701.940+16.83%1.38M17.98M11.68011.53013.53011.620840.75M611.26M62.42M45.38M+44.37%+16.22%+10.41%-8.43%-11.38%+80.56%+25.89%--3.05%LossLoss16.57%Biotechnology
TILInstil Bio
29.70004.2700+16.79%150.89K4.34M25.530025.430030.450025.5300193.82M71.00M6.53M2.39M+27.14%-5.83%-13.36%+101.49%+177.57%+338.31%+289.76%--6.31%LossLoss19.35%Biotechnology
PULMPulmatrix
7.3401.050+16.69%466.21K3.33M6.5006.2907.5206.50026.81M26.81M3.65M3.65M+30.60%+106.18%+236.70%+258.05%+272.59%+256.31%+294.62%--12.77%LossLoss16.22%Biotechnology
TRAWTraws Pharma
4.7420.652+15.93%8.59K37.79K4.1004.0904.7504.10014.35M10.82M3.03M2.28M+5.60%-16.08%-4.60%-48.05%-70.82%-72.47%-74.56%--0.38%LossLoss15.89%Biotechnology
IKTInhibikase Therapeutics
2.73000.3700+15.68%293.86K779.45K2.40002.36002.86472.3900183.44M27.95M67.19M10.24M+37.88%+12.81%-1.44%+102.22%+61.54%+191.20%+114.96%--2.87%LossLoss20.11%Biotechnology
ACETAdicet Bio
1.1100.150+15.59%1.31M1.35M0.9900.9601.1200.96091.47M68.93M82.40M62.10M+14.78%-2.63%-18.38%-26.49%-17.78%-5.93%-41.27%--2.11%LossLoss16.66%Biotechnology
XCURExicure
31.99004.3000+15.53%512.90K16.42M27.580027.690036.000026.471269.49M36.99M2.17M1.16M+185.88%+1071.79%+1120.99%+1707.34%+1515.25%+1287.85%+1002.53%--44.36%LossLoss34.41%Biotechnology
UNCYUnicycive Therapeutics
0.67790.0878+14.88%3.79M2.56M0.61470.59010.73500.605070.36M47.66M103.80M70.31M+33.08%+41.79%+32.92%+100.56%-12.61%+34.93%-21.87%--5.39%LossLoss22.03%Biotechnology
CRVOCervoMed
9.1551.175+14.72%586.64K5.55M8.5407.98010.2908.26075.56M37.37M8.25M4.08M-5.03%-18.40%-34.98%-45.96%-52.29%-6.58%+19.99%--14.37%LossLoss25.44%Biotechnology
NKTXNkarta
2.9900.380+14.56%1.85M5.35M2.6502.6102.9952.650211.00M131.22M70.57M43.89M+19.60%+2.75%-10.21%-44.53%-54.70%+13.26%-54.70%--4.21%LossLoss13.22%Biotechnology
IVVDInvivyd
0.6740.084+14.27%2.05M1.44M0.6240.5900.7820.62380.65M30.86M119.62M45.77M-12.22%-24.25%-25.92%-20.69%-60.11%-55.93%-82.89%--4.49%LossLoss26.83%Biotechnology
IOBTIO Biotech
0.8500.102+13.64%791.77K645.16K0.7400.7480.8700.72156.00M23.67M65.88M27.84M+3.58%-12.38%-33.59%-11.98%-31.06%-27.35%-54.79%--2.84%LossLoss19.92%Biotechnology
EPRXEupraxia Pharmaceuticals
3.4500.400+13.11%5.70K18.89K3.0603.0503.5603.060122.90M88.23M35.62M25.57M-4.17%+21.48%+38.00%+40.82%+28.73%+6.48%+6.48%--0.02%LossLoss16.39%Biotechnology
ALLKAllakos
1.0100.116+12.95%352.31K350.00K0.8840.8941.0400.88490.24M60.31M89.34M59.71M+7.54%-22.31%-18.55%+53.03%-12.93%-54.50%-63.00%--0.59%LossLoss17.49%Biotechnology
VSTMVerastem
4.57000.5200+12.84%2.80M12.62M4.11004.05004.77004.0800203.39M138.83M44.51M30.38M+20.58%+11.46%+22.19%+87.30%+34.41%-33.67%-43.86%--9.22%LossLoss17.04%Biotechnology
BCTXBriaCell Therapeutics
0.8420.095+12.74%1.14M903.55K0.7560.7470.8420.74030.48M26.66M36.18M31.65M+39.85%+40.29%+9.40%+16.48%-39.40%-84.08%-85.60%--3.60%LossLoss13.69%Biotechnology
GNTAGenenta Science
5.3800.600+12.55%12.44K63.27K4.6704.7805.6804.60198.40M60.41M18.29M11.23M+14.47%+7.60%+0.84%+25.41%+63.03%+3.46%+8.69%--0.11%LossLoss22.57%Biotechnology
1
2
3
4
5
23
News
Applied Therapeutics Says FDA Unable To Approve Govorestat NDA Due To Clinical Deficiencies; Expects To Submit An NDA Early In Q1 Of 2025
Applied Therapeutics Receives Complete Response Letter From U.S. FDA Regarding New Drug Application For Govorestat For Classic Galactosemia
TC BioPharm Plc Files For Mixed Shelf Of Up To $100M
Wednesday Ends With Index Decline | Wall Street Today
Titan Pharmaceuticals Gets Non-Compliance Notice From Nasdaq
12 Health Care Stocks Moving In Wednesday's Pre-Market Session
Comments
$Biotechnology (LIST2069.US)$
if yes, which company will be the first to crash?
if yes, which company will be the first to crash?
My opinion it will go down 25 basis points, and make rally for $Biotechnology (LIST2069.US)$ and $Technology (LIST20763.US)$ sector...
8
hhh, reenter.. I should have stopped daytrade better swing for biotech, easier to get gain if got FDA approval...
$Biotechnology (LIST2069.US)$
$Iovance Biotherapeutics (IOVA.US)$
$Biotechnology (LIST2069.US)$
$Iovance Biotherapeutics (IOVA.US)$
2
$Amylyx Pharmaceuticals (AMLX.US)$
I like the growth, no position. this kind of growth only happens for several industry currently technology or biotech, $Nu Holdings (NU.US)$ , $GigaCloud Technology (GCT.US)$ , $Futu Holdings Ltd (FUTU.US)$ ... kind of similar in revenue and eps chart...
kind of my type. three consecutive quarters profitable. turnaround is happening. grow from minus eps to profitable.
still undervalue if it's measured by PB industry, CR High. some products are also still haven't appro...
I like the growth, no position. this kind of growth only happens for several industry currently technology or biotech, $Nu Holdings (NU.US)$ , $GigaCloud Technology (GCT.US)$ , $Futu Holdings Ltd (FUTU.US)$ ... kind of similar in revenue and eps chart...
kind of my type. three consecutive quarters profitable. turnaround is happening. grow from minus eps to profitable.
still undervalue if it's measured by PB industry, CR High. some products are also still haven't appro...



7
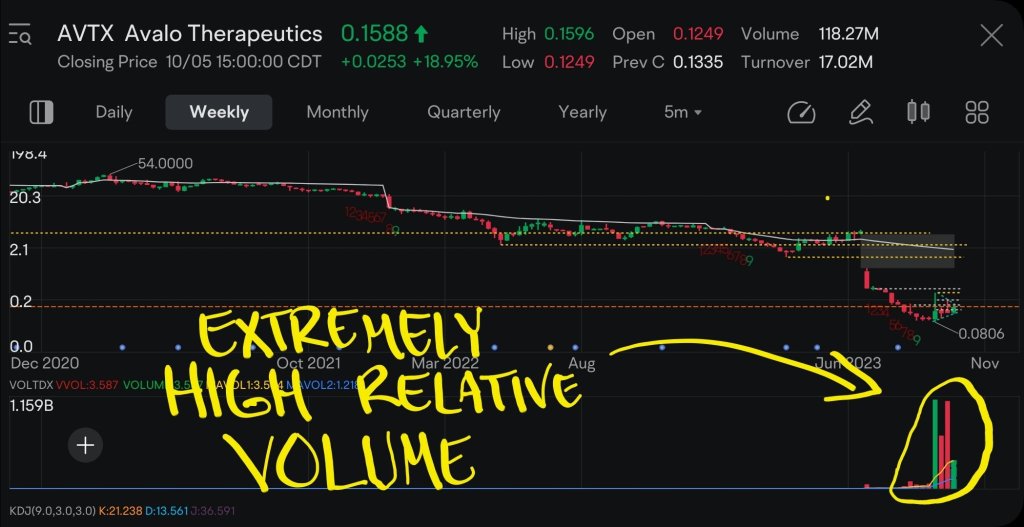
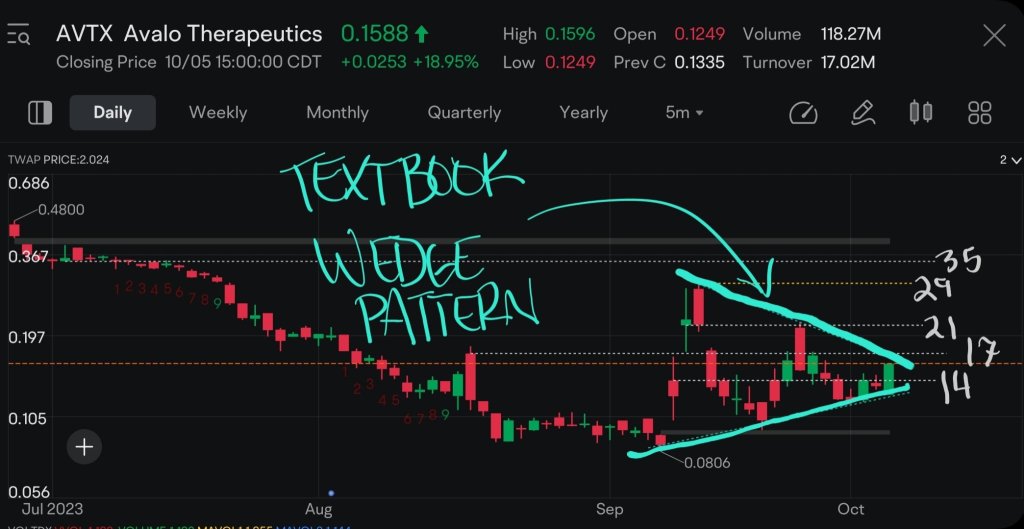
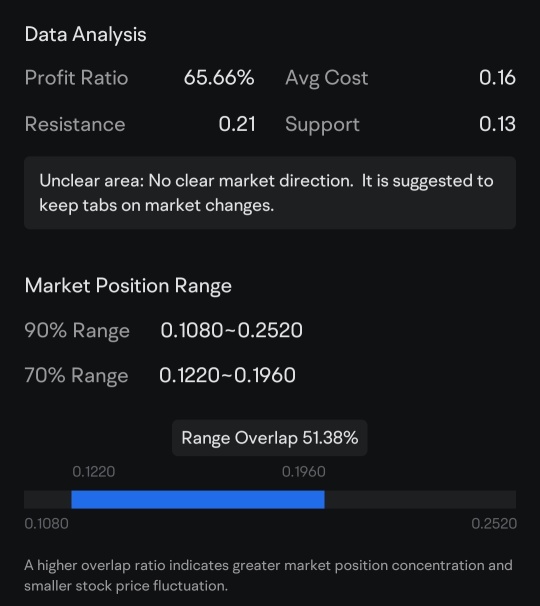
$Avalo Therapeutics (AVTX.US)$
Poor Performance So Far
Over the past few weeks, we have seen a lot of unusual activity in this ticker symbol. If there is anyone out there who knows why activity has picked up in AVTX, then please enlighten me.
Over the past few years, putting money into this company has been losing bet. Any stock that is in a long-term downtrend is usually something that I would stay away from.
Intriguing Technical Developmemts
Over the past few weeks,...
Poor Performance So Far
Over the past few weeks, we have seen a lot of unusual activity in this ticker symbol. If there is anyone out there who knows why activity has picked up in AVTX, then please enlighten me.
Over the past few years, putting money into this company has been losing bet. Any stock that is in a long-term downtrend is usually something that I would stay away from.
Intriguing Technical Developmemts
Over the past few weeks,...
+4
9
3
Read more
